All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
BMS-986158 in combination with JAKi: An early analysis of disease modification in myelofibrosis
Do you know... What percentage of patients with treatment-naïve MF experienced a ≥20% reduction in JAK2v617F VAF reduction?
BMS-986158 is a selective oral bromodomain and extra-terminal motif inhibitor currently under evaluation in combination with the Janus kinase inhibitors (JAKi) ruxolitinib or fedratinib in patients with treatment-naïve (TN) and relapsed/refractory (R/R) myelofibrosis (MF), as part of the phase 1b/2 CA011-023 (NCT04817007) study.
JAKi are the current standard of care for MF. The use of bromodomain and extra-terminal motif inhibitor in combination has been shown to reduce proinflammatory signals and disease burden preclinically – a key differentiator from the standard of care in MF.
During the 65th American Society of Hematology (ASH) Annual Meeting, Lee-Hoflich, et al. presented an analysis of the early disease-modifying properties of BMS-986158 in combination with JAKi. Here, we summarize the key points.
Check out our top abstracts presented at the 65th ASH Annual Meeting and Exposition here.
Design1
A summary of the trial design and key eligibility criteria are provided in Figure 1.
Figure 1. CA011-023 study design and eligibility criteria*
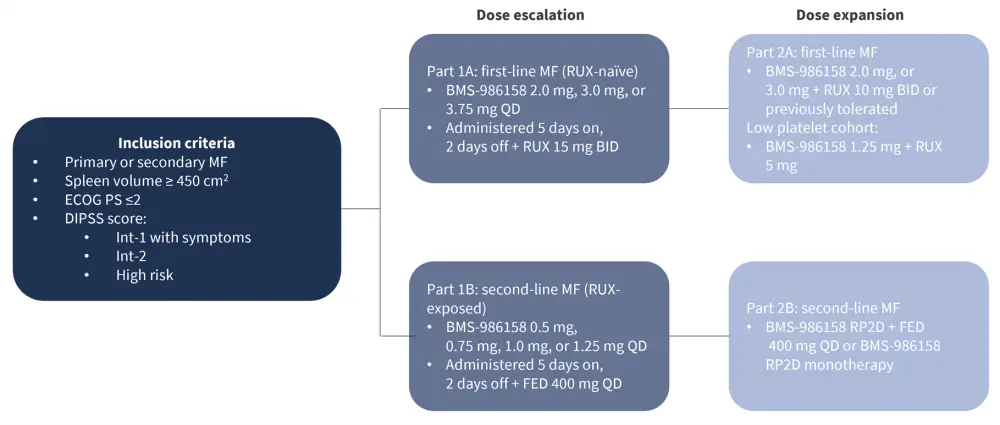
BID, twice a day; DIPSS, Dynamic International Prognostic Scoring System; ECOG PS, Eastern Cooperative Oncology Group performance status; FED, fedratinib; Int, intermediate; MF, myelofibrosis; QD, daily; RP2D, recommended phase II dose; RUX, ruxolitinib.
*Adapted from Lee-Hoflich, et al.1
In this analysis, exploratory measures of disease modification included:
- measurement of longitudinal JAK2 VAF;
- evaluation of the bone marrow microenvironment;
- megakaryocyte cluster density; and
- modulation of circulatory cytokines and growth factors.
Results1
- In total, 80% of patients with TN MF (n = 10) and 57% of patients with R/R MF (n = 7) experienced a ≥20% JAK2v617F VAF reduction.
- Most patients reached persistent JAK2v617F VAF reduction at 48 weeks.
- A reduction in fibrosis was observed in both TN and R/R MF, positively associated with an increasing dose level of BMS-986158.
- Overall, 45% of patients with TN MF and 20% with R/R MF achieved a Grade ≥1 bone marrow fibrosis (BMF) reduction, associated positively with increased dose levels.
A summary of Grade ≥1 BMF reductions for TN and R/R patients are outlined in Tables 1 and 2, respectively.
Table 1. Bone marrow fibrosis reduction in patients with treatment-naïve myelofibrosis*
|
BID, twice daily; MF, myelofibrosis; QD, daily; RUX, ruxolitinib. |
|||
|
BMS-986158 QD + RUX 15 mg BID, % (unless otherwise specified) |
Grade ≥1 reduction |
Stabilized (maintained at MF 1 or MF 2) |
Progressed (increased MF score or at highest grade MF 3) |
|---|---|---|---|
|
2.0 mg |
0 |
100 |
0 |
|
3.0 mg |
50 |
25 |
25 |
|
3.75 mg |
100 |
0 |
0 |
Table 2. Bone marrow fibrosis reduction in patients with relapsed/refractory myelofibrosis*
|
BID, twice daily; FED, fedratinib; MF, myelofibrosis; QD, daily. |
|||
|
BMS-986158 QD + FED 400 mg, % (unless otherwise specified) |
Grade ≥1 reduction |
Stabilized (maintained at MF 1 or MF 2) |
Progressed (increased MF score or at highest grade MF 3) |
|---|---|---|---|
|
0.5 mg |
0 |
0 |
100 |
|
0.75 mg |
0 |
25 |
75 |
|
1.0 mg |
50 |
50 |
0 |
Conclusion
Data from this biomarker analysis highlights potential disease-modifying properties of combination BMS-986158 + JAKi in both TN and R/R MF. In this small cohort, most patients reached ≥20% JAK2v617F VAF reduction, as the dose levels increased. This suggests that this combination could be a feasible treatment option for further clinical trials.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


