All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
EBMT/ELN updated recommendations for allo-HSCT in myelofibrosis
Do you know... According to the recently revised EBMT/ELN recommendations on allogeneic hemopoietic stem cell transplantation, what prognostic model scores indicate the need for immediate transplantation?
In 2015, the European Group for Blood and Marrow Transplantation (EBMT) and European LeukemiaNet (ELN) co-developed consensus-based guidelines for the indication and management of allogeneic hemopoietic stem cell transplantation (allo-HSCT) in myelofibrosis (MF). Since then, new therapies and combination treatments, improvements in patient/donor selection, conditioning regimens, and posttransplant supportive care have enabled an increased proportion of patients to access the potentially curative option of stem cell transplantation. However, these treatment advances have created new challenges, with the optimal management strategies for MF still unclear. To address this, the EBMT/ELN working group has reviewed data from 2015–2022 and revised the 2015 recommendations to optimize the use of allo-HSCT in patients with MF. Here, we summarize the key updates.
Study design1
- Data was reviewed by a 26-member expert panel
- Recommendations were consensus-based, and not derived from systematic reviews and evidence grading
- The expert panel agreed on seven areas of major concern and rank-ordered 18 key clinical questions
- Each recommendation required 80% of votes in favor for final inclusion
Patient selection and timing1
- Over the past two decades, several prognostic tools have been developed for primary MF
- These models have been extremely useful in predicting outcomes in patients with key high-risk mutations and clinical features
- Recommendations for allo-HSCT according to prognostic model scores are shown in Figure 1
Figure 1. Prognostic model scores and allo-HSCT recommendations*
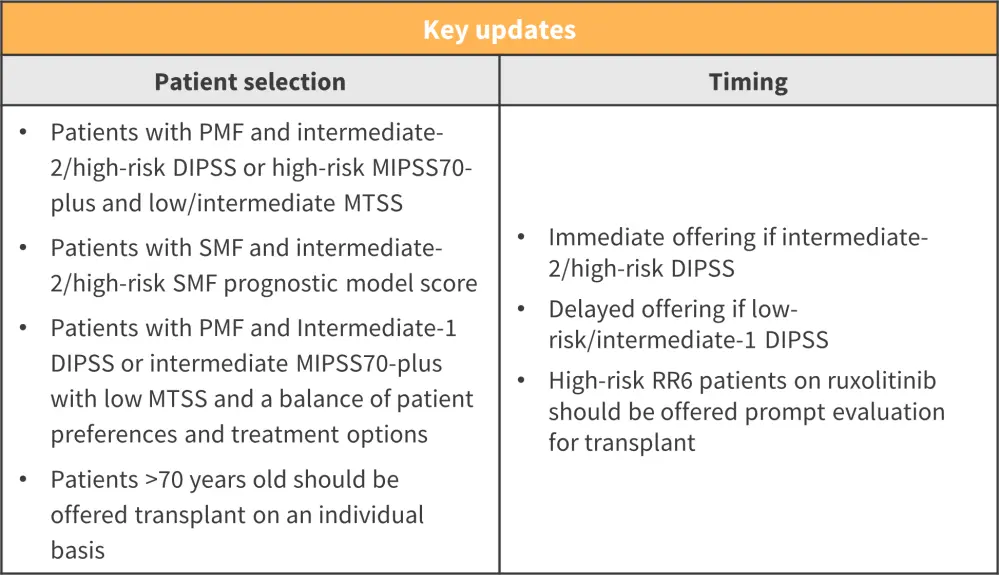
Allo-HSCT, allogeneic hemopoietic stem cell transplantation; DIPSS, Dynamic International Prognostic Scoring System; MIPSS70, Mutation-Enhanced International Prognostic Scoring System 70; MTSS, Myelofibrosis Transplant Scoring System; PMF, primary myelofibrosis; RR6, response to ruxolitinib after 6 months model; SMF, secondary myelofibrosis.
*Adapted from Kroger, et al.1
Pretransplant management1
- Pretransplant management techniques are highly important to ensure the best outcomes
- Management techniques recommended for patients before transplant are shown in Figure 2
Figure 2. Pretransplant management recommendations*
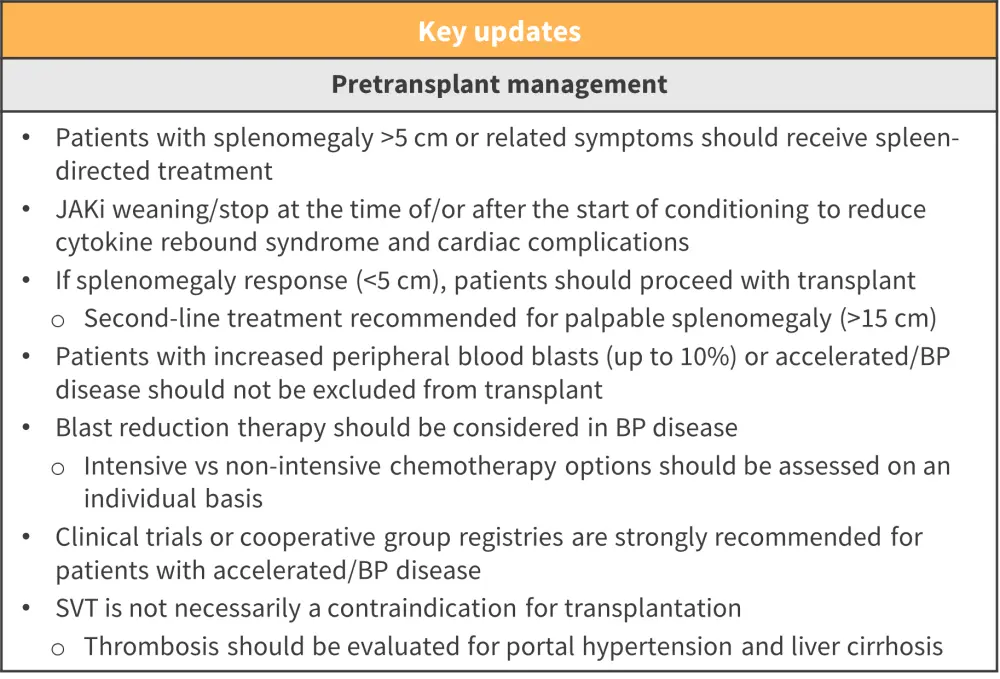
BP, blast phase; JAKi, Janus kinase inhibitor; SVT, splanchnic vein thrombosis.
*Adapted from Kroger, et al.1
Donor selection, stem cell source/dose, and conditioning regimen1
- Donor type is considered a significant predictor of transplant-related mortality
- As a result of no current randomized comparisons, peripheral blood remains the preferred stem cell source compared with bone marrow grafts
- Conclusive evidence surrounding optimal conditioning regimens remains scarce
- Recommendations surrounding these clinical areas are highlighted in Figure 3
Figure 3. Recommendations for donor selection, stem cell source/dose, and conditioning regimen*
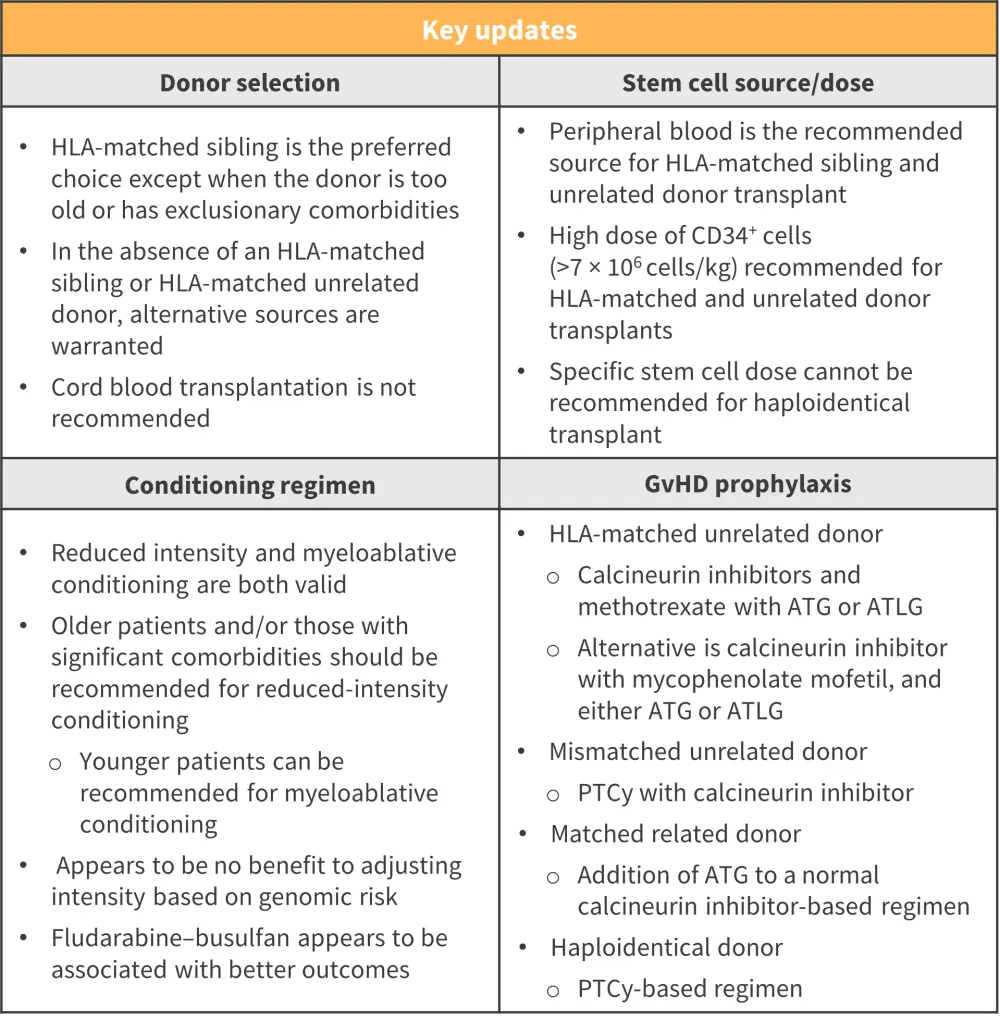
ATG, anti-thymocyte globulin; ATLG, anti-T lymphocyte globulin; GvHD, graft-versus-host disease; HLA, human leukocyte antigen; PTCy, posttransplant cyclophosphamide.
*Adapted from Kroger, et al.1
Posttransplant management1
- Posttransplant management remains another key area of clinical importance
- Effective management at this stage can reduce the risk of poor graft function, molecular relapse, and the need for a second transplant
- Recommendations for posttransplant management are shown in Figure 4
Figure 4. Recommendations for posttransplant management*
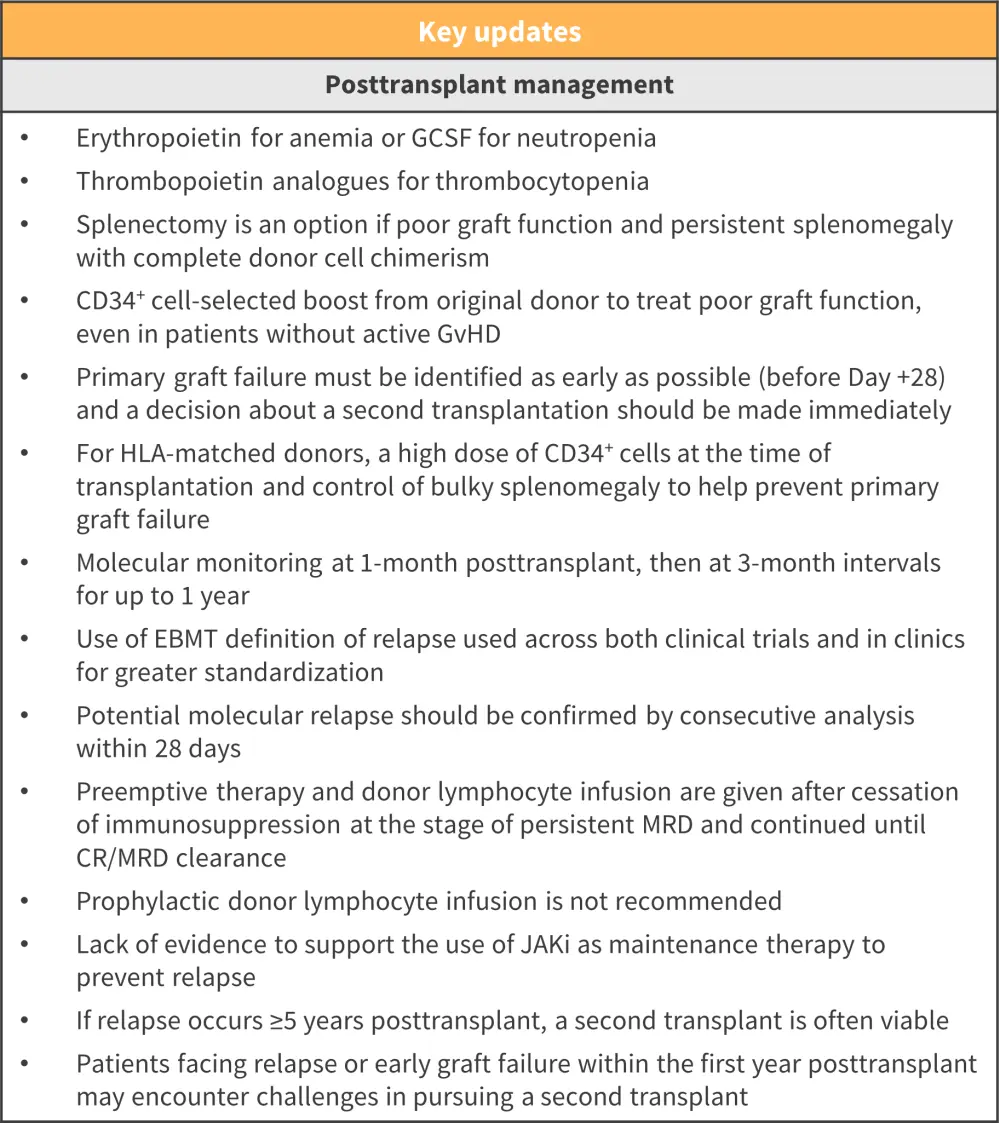
CR, complete remission; EBMT, European Group for Blood and Marrow Transplantation; GCSF, granulocyte colony-stimulating factor; GvHD, graft-versus-host disease; HLA, human leukocyte antigen; JAKi, Janus kinase inhibitor; MRD, measurable residual disease.
*Adapted from Kroger, et al.1
Conclusion1
There is a lack of evidence from randomized trials regarding the optimal indication and management of allo-HSCT in MF; current recommendations are limited by retrospective analyses. However, the expert panel of the EBMT/ELN International Working Group comprises members from leading clinical centers, offering updated recommendations to assist clinicians and patients in optimizing allo-HSCT in MF. These guidelines aim to improve clinical outcomes and guide future processes in the absence of evidence-based guidance.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




