All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Educational theme | Treating systemic mastocytosis
Within this Educational Theme, our second article focuses on the treatment of systemic mastocytosis (SM), a rare hematologic malignancy where abnormal neoplastic mast cells accumulate in extra-cutaneous organs. The majority of adult patients with SM harbor gain-of-function mutations in the KIT tyrosine kinase domain, especially the D816V domain. Risk stratification is a key factor in informing treatment decisions, and treatment options differ from observation (only for low-burden disease) through to cytoreduction (for advanced disease).1
Here, a treatment overview and summary of latest data on KIT-targeting tyrosine kinase inhibitors (TKIs) are provided. Read our previous article on the disease overview summarizing pathogenesis, diagnostic criteria, and risk stratification here.
Treating SM
The treatment of SM in adult patients is individualized. The treatment goal in low-burden disease, i.e., indolent SM (ISM) or smoldering SM (SSM), is mainly to improve symptoms, while novel therapeutic options, e.g., TKIs, are considered in more advanced disease. For the treatment of ISM/SSM, midostaurin has been shown to have potential in controlling severe mast cell mediator release syndrome in patients who fail conventional anti-mediator therapy. It is also important to enroll patients with SM in clinical trials, to explore new therapeutic options and support drug development. Figure 1 outlines the treatment algorithm for SM.1,2
Figure 1. Treatment algorithm of SM*
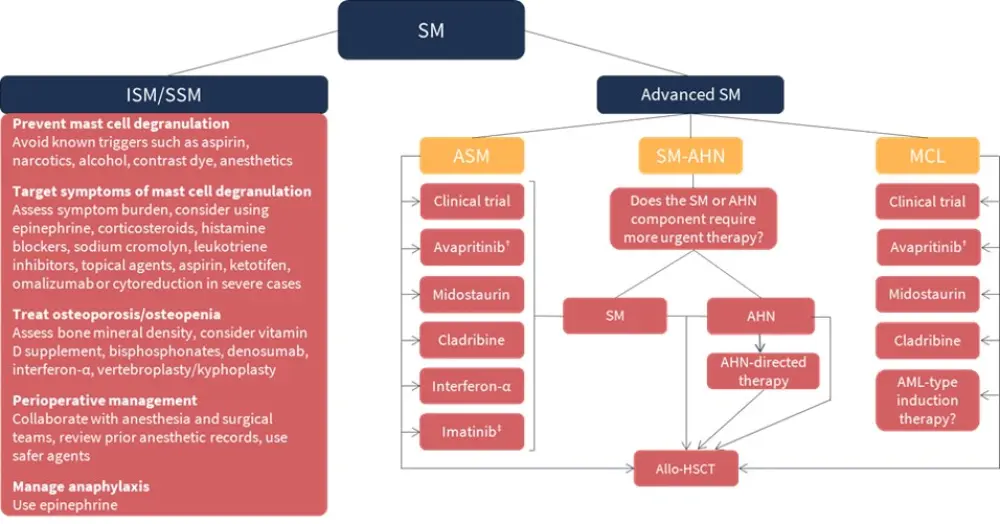
AHN, associated hematologic neoplasm; Allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; ASM, aggressive SM; ISM, indolent SM; MCL, mast cell leukemia; SM, systemic mastocytosis; SM-AHN, SM with an associated hematologic neoplasm; SSM, smoldering SM.
*Adapted from Pardanani1 and Gotlib2
†If platelets ≥50 × 109/L.
‡If KIT D816V mutation is negative or unknown.
Symptom-directed therapy
Therapy in all patients with SM, regardless of disease burden, should be guided by their disease-related symptoms, such as the treatment or prevention of mast cell degranulation, symptomatic skin disease, and osteoporosis/osteopenia (Figure 1). Anti-mediator therapy, e.g., H1/H2 antihistamines, should be considered as needed.1,2
Allogeneic hematopoietic stem cell transplantation (allo-HSCT)
Selected patients with SM may benefit from allo-HSCT to prolong survival, and this can be used in patients with relapsed/refractory aggressive SM (ASM). In SM with an associated hematologic neoplasm (SM-AHN), allo-HSCT can be used to treat AML as an aggressive AHN component, or as salvage therapy in disease progression following a reassessment of dominant component (SM vs AHN).1 In patients with advanced SM, a retrospective analysis found that overall survival (OS) at 3 years following allo-HSCT was 57% (SM-AHN: 74%; ASM: 43%; mast cell leukemia [MCL]: 17%). Adverse factors for OS included MCL and lower response for reduced-intensity conditioning compared to myeloablative conditioning.2
Conventional therapeutic approaches
Conventional cytoreductive therapies include cladribine, interferon, and hydroxyurea; however, their efficacy in eliminating neoplastic mast cells or alleviating disease burden varies across patients.3
Cladribine
Cladribine (chlorodeoxyadenosine, 2-CDA) has demonstrated therapeutic activity in all SM subtypes in historical clinical trials, although the sample size was small in all studies. Response rates were greater in patients with low-burden disease compared with advanced disease. The most common Grade 3/4 adverse events (AEs) reported were cytopenias and opportunistic infections.1
At the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, Ayalew Tefferi presented the latest data on cladribine from 42 patients with advanced SM (n = 22) and ISM/SSM (n = 20).4 Overall response rate (ORR) in patients with advanced SM was 77% (ASM: 88%; SM-AHN: 69%) with a median time to response of 3.7 months (range, 0.4–9). Among responders, 45% had major response and 32% had partial response. Median duration of response (DoR) was 6 months (range, 1-67). The safety profile was consistent with previous studies; 36% of patients experienced Grade 3/4 cytopenias. In the ISM cohort, ORR was 70% with 60% of patients having complete or major regression of symptoms or urticaria pigmentosa; median time to response was 8.5 months (range, 1–98). Treatment-emergent AEs included Grade 3/4 cytopenia (15%) and lymphopenia (30%).4
Cladribine can be used as first-line treatment in rapid mast cell debulking, and as salvage treatment in patients progressing following interferon-α, midostaurin, or other cytoreductive therapy.1
Interferon-α
Interferon-α is a common treatment for myeloproliferative neoplasms (MPN) and has been used in SM since the mid-1980s. It has been associated with improvements in cutaneous, hematologic, gastrointestinal, and systemic symptoms, organ enlargement, and osteoporosis. A pegylated form is available and can be combined with steroids, i.e., prednisone, to improve tolerability and response. Long-term treatment may be required in order to achieve a response.1,3
Hydroxyurea
Hydroxyurea has demonstrated myelosuppressive activity in SM; however, it lacks selective anti-mast cell activity.1
Contemporary therapeutic options
Recently, small molecule kinase inhibitors targeting KIT mutations have been shown to result in clinical and histologic responses. The TKIs imatinib, midostaurin, and, very recently, avapritinib are currently approved for the treatment of SM.1,2
Imatinib
Imatinib mesylate was the first TKI to be investigated as a treatment for mastocytosis. It inhibits ATP binding, and prevents phosphorylation, secondary growth receptor activation and downstream signaling, in addition to inhibiting activity in PDGFR and KIT.3 It was approved by the U.S Food and Drug Administration (FDA) in 2006 for the treatment of ASM in patients without the KIT D816V mutation or with unknown KIT mutational status.5 Historical data leading to its approval showed improvements in disease burden with imatinib in patients with symptomatic SM and who were KIT D816V-negative, whereas responses were low in patients with KIT-positive disease. Based on these data, imatinib is thought to have a limited role in the treatment of patients with SM, as the majority harbor the KIT D816V mutation.1,3
Midostaurin
Midostaurin is a multi-kinase type I TKI which inhibits autophosphorylation of c-KIT and activation of further downstream processes.3 It was approved by the FDA6 and European Medicines Agency (EMA)7 for the treatment of adult patients with ASM, SM-AHN, or MCL in 2017. In the Global D2201 midostaurin trial (NCT00782067), which led to the FDA approval, the ORR was 60%, with 45% of patients demonstrating a major response and 15% of patients a partial response. Across SM types, ORR was 75% (ASM), 58% (SM-AHN), and 50% (MCL); median DoR was 24.1 months.2
Tolerability is the main disadvantage of midostaurin, with patients often needing a reduction in dose due to gastrointestinal events, which also require management and antiemetic prophylaxis.3 Despite this fact, midostaurin can be considered both as a first-line therapy, especially in MCL, and as salvage treatment in patients progressing following interferon-α, cladribine, or other cytoreductive therapy.1
Avapritinib
Avapritinib is an oral type 1 TKI and a selective inhibitor of activation-loop mutants of KIT including D816V.3 The FDA granted approval for avapritinib in the treatment of adult patients with advanced SM including ASM, SM-AHN, and MCL, based on favorable findings in the phase I EXPLORER (NCT02561988) and phase II PATHFINDER (NCT03580655) trials.3,8 Table 1 provides response rates noted in the EXPLORER study, and the latest results from the PATHFINDER trial are presented here.
Briefly, in the EXPLORER study1,2:
- 92% of patients had ≥50% reduction in bone marrow mast cell burden
- 99% of patients had ≥50% reduction in serum tryptase levels
- 82% of patients had ≥35% reduction in spleen volume
- 80% of patients had ≥50% reduction in KIT D816V variant allele frequency (VAF), with 30% showing undetectable VAF
- Median OS was not reached in the overall advanced SM safety population (median duration of follow-up was 23 months)
- Estimated 24-month OS rate was 76% (vs 53% with midostaurin)
- Avapritinib was well tolerated
- Reasons for treatment discontinuation included disease progression (33%), AEs (33%), and inadequate response (17%)
- 8% of patients experienced non-traumatic intracranial bleeding resulting in a protocol change to include dose modifications for the management of severe thrombocytopenia
Table 1. ORR by modified IWG-MRT-ECNM criteria in the EXPLORER study*
|
ASM, aggressive systemic mastocytosis; CR, complete response; CRh, complete response with partial hematologic recovery; IWG-MRT-ECNM, International Working Group-Myeloproliferative Neoplasms Research and Treatment-European Competence Network on Mastocytosis; MCL, mast cell leukemia; ORR, overall response rate; PR, partial response; SD, stable disease; SM-AHN, systemic mastocytosis with an associated hematologic neoplasm. |
||||||
|
Best confirmed central response (%) |
All evaluable |
ASM |
SM-AHN |
MCL |
Midostaurin-naïve |
Post midostaurin |
|---|---|---|---|---|---|---|
|
ORR |
75 |
100 |
76 |
69 |
83 |
59 |
|
CR or CRh |
36 |
67 |
38 |
23 |
44 |
18 |
|
PR |
34 |
33 |
35 |
31 |
33 |
35 |
|
SD |
23 |
0 |
22 |
31 |
17 |
35 |
In the PATHFINDER trial3:
- 88% of patients had ≥50% reduction in mast cell burden in the bone marrow
- 93% of patients had ≥50% reduction in serum tryptase levels
- There was a significant reduction in KIT D816V VAF in patients with SM-AHN
- 84% of patients remained on treatment, 5% of patients discontinued treatment due to AEs
- Most common Grade 3/4 AEs included thrombocytopenia, anemia, and neutropenia
Avapritinib is also under investigation in the phase II PIONEER study (NCT03731260) for the treatment of patients with ISM. Based on available data from 39 patients, avapritinib was well tolerated at all doses, and no Grade 4 or 5 AEs were reported. Compared to placebo, avapritinib demonstrated symptom alleviation at an early stage (30% mean symptom reduction was recorded by Week 16). At the 25 mg dose level, there were improvements in mast cell burden, serum tryptase level, and allelic burden; therefore, this was selected as the recommended phase 2 dose for the ongoing trial.3
DCC-2618
DCC-2618 is an investigational, potent type II switch pocket control inhibitor of exon 17 KIT mutations that are resistant to conventional TKIs. It is currently under investigation in a phase I study (NCT02571036) including patients with ASM, SM-AHN, and MCL.1
Conclusion
The development of potent and selective TKIs has revolutionized treatment strategies for patients with mastocytosis. Clinical trials investigating KIT-targeting agents have revealed that partial or complete normalization of mast cell burden, serum tryptase levels, spleen volume, organ damage, and skin symptoms is achievable. Avapritinib is considered the most effective TKI, with promising results obtained in patients with ASM, and clinical research continues in this therapeutic area. During his presentation at the 9th Annual Meeting of the Society of Hematologic Oncology (SOHO 2021), Jason Gotlib proposed that key clinical areas requiring investigation included understanding the disease-modifying effects of avapritinib, the potential of next-generation sequencing (NGS) in identifying potential mechanisms of resistance, KIT inhibitor sequencing with AHN-directed therapy in SM-AHN, and consideration of the role of KIT inhibition before and after transplant.2
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

