All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
EXPAND: A phase Ib clinical trial of ruxolitinib for patients with MF and low platelet counts
Ruxolitinib is a Janus kinase-1 and -2 inhibitor with U.S. Food and Drug Administration (FDA) approval for the treatment of primary and secondary intermediate and high-risk myelofibrosis (MF).1 The MPN Hub has previously reported on the benefits of ruxolitinib for patients in the real-world setting, including significant reduction in the risk of mortality and improvements in overall survival.
FDA approval for ruxolitinib was received in 2011,1,2 based on the COMFORT trials (covered on MPN Hub here), in which ruxolitinib demonstrated efficacy in the reduction of splenomegaly and in improving symptoms and survival in patients with MF. Treatment was well tolerated, with side effects easily managed with simple dose adjustment.2,3
The COMFORT trials used platelet count as an indicator of both toxicity and effective treatment to guide dosing.2,3 As MF progresses, platelet counts invariably reduce in patients as they experience progressive disease-related bone marrow failure and increasing splenic platelet consumption.2,3 Eventually, thrombocytopenia can become a dose-limiting comorbidity for patients who could benefit from treatment with ruxolitinib and can even preclude treatment initiation.2,3
In this article, the MPN Hub summarizes data from the EXPAND trial (NCT01317875), which were published in Therapeutic Advances in Hematology in July 2022.3 Based on results from the trial, Guglielmelli and colleagues suggest the optimal starting dose of ruxolitinib for patients with a platelet count of 50 to <100 × 109/dL.3
Study design
EXPAND was a single-center phase Ib, open-label, dose-finding study, which ran from March 2011 to December 2019. The study design (Figure 1) featured a 24-week dose escalation and safety phase, which collected efficacy and toxicity data to establish a maximum safe starting dose (MSSD) of ruxolitinib. Patients subsequently entered an extended follow-up period to determine the long-term data relating to the established MSSD.
Upon enrolment, patients were assigned to one of two cohorts (Stratum 1 or 2), according to their baseline platelet count:
- Stratum 1: Platelet count, 75–100 × 109/dL
- Stratum 2: Platelet count, 50–74 × 109/dL
Figure 1. EXPAND trial study design*
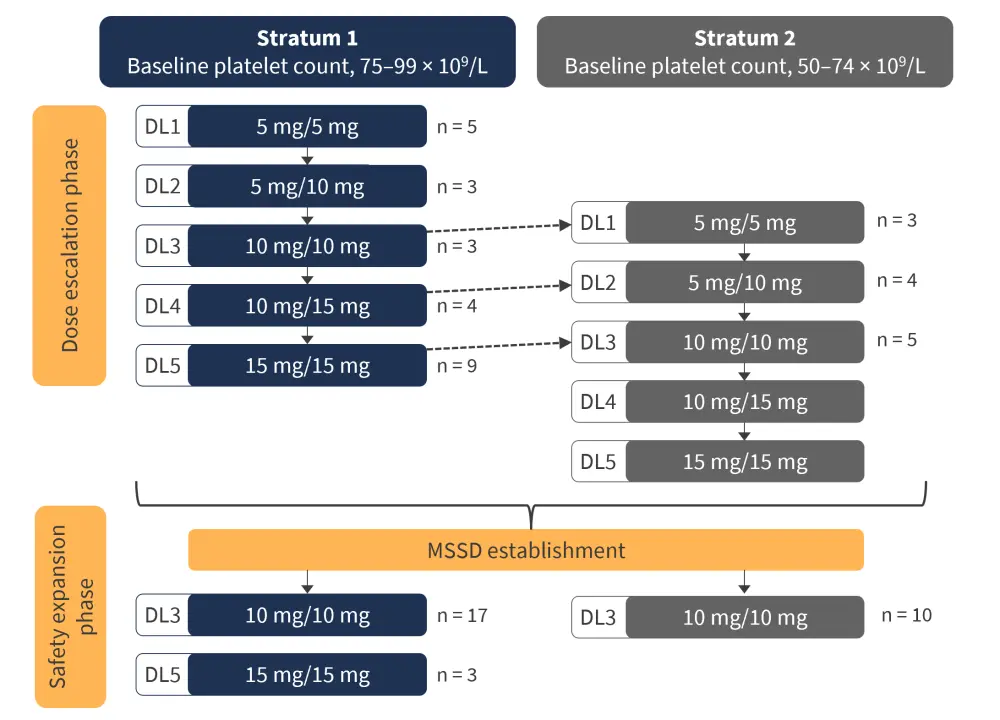
DL, dose level; MSSD, maximum safe starting dose.
*Adapted from Vannucchi, et al.2
All patients were aged 18 years or older, had a diagnosis of intermediate-1, intermediate-2, or high-risk MF (including primary MF, primary polycythemia-MF, or primary essential thrombocytopenia-MF), and had a palpable spleen ≥5 cm from the costal margin.
The study had predetermined endpoints, including:
- Primary endpoint:
- Establishment of MSSD for each stratum
- Secondary endpoints:
- Adverse events, including frequency, duration, and severity
- Symptoms, assessed through patient self-report using the Myelofibrosis Symptom Assessment Form
- Splenic response, defined as ≥50% reduction in spleen size
- Correlation between serum ruxolitinib levels and clinical measures (e.g., platelet count and spleen size)
Results
Of the 69 enrolled patients, 38 patients received ruxolitinib at the MSSD, with 20 patients in Stratum 1 and 18 patients in Stratum 2. Demographic and clinical information can be seen in Table 1.
Table 1. Baseline characteristics of patients in Stratum 1 and Stratum 2*
|
Int, intermediate; IWG, international working group; JAK2, Janus kinase 2; MF, myelofibrosis; PET, primary essential thrombocytopenia; PMF, primary myelofibrosis; PPV, primary polycythaemia vera. |
||
|
Characteristic, % (unless stated otherwise) |
Stratum 1 |
Stratum 2 |
|---|---|---|
|
Median age (range), years |
64.5 (27–81) |
66.5 (46–86) |
|
Age ≥65 years |
50.0 |
61.1 |
|
Sex |
||
|
Male |
40.0 |
61.1 |
|
Female |
60.0 |
38.9 |
|
MF subtype |
||
|
PMF |
90.0 |
72.2 |
|
PPV-MF |
5.0 |
16.7 |
|
PET-MF |
5.0 |
11.1 |
|
Median spleen length (range), cm |
10.5 (5–25) |
12.0 (4–33) |
|
JAK2 mutation |
||
|
Positive |
95.0 |
66.7 |
|
Negative |
5.0 |
27.8 |
|
Not assessed |
0.0 |
5.6 |
|
IWG risk at screening |
||
|
Int-1 |
50.0 |
11.1 |
|
Int-2 |
40.0 |
44.4 |
|
High risk |
10.0 |
44.4 |
|
Median time since initial diagnosis (range), months |
19.1 (1.7–190.0) |
39.5 (1.3–335.6) |
|
Median hemoglobin (range), g |
107.5 (51–155) |
104.0 (58–150) |
|
Median baseline platelet count (range), ×109/dL |
84.0 (75–99) |
57.5 (50–74) |
A ≥50% reduction in spleen size was observed in both Stratum 1 (50%) and Stratum 2 (67%) at any point in treatment, with respective rates of 40% and 37.5% at 24 weeks, and 50% and 66.7% at 48 weeks. The median duration of ruxolitinib treatment was 134.3 weeks, with most patients experiencing a dose reduction at some point over the course of treatment (equivalent across both strata).
Patient symptoms (measured using the MF total symptom score) improved by a median of −5.3 (range, −7 to 27) in Stratum 1 and a median of 0.1 (range, −29 to 8) in Stratum 2, with an improvement across all symptoms except for bone/muscle pain.
Within Stratum 1, 50% of patients completed their planned treatment course, compared with 16.7% of patients with a lower platelet counts at baseline (Stratum 2). Reasons for discontinuation of treatment can be seen in Table 2, with adverse events being the most common causative factor (Stratum 1, 10%; Stratum 2, 33.3%).
Table 2. Treatment outcomes of patients recruited to EXPAND clinical trial*
|
*Adapted from Guglielmelli, et al.3 |
||
|
Treatment outcome, % |
Stratum 1 |
Stratum 2 |
|---|---|---|
|
Completed treatment period |
50.0 |
16.7 |
|
Discontinued from treatment period |
50.0 |
83.3 |
|
Reason for discontinuation |
|
|
|
Reason for discontinuation |
||
|
Adverse event |
10.0 |
33.3 |
|
Physician decision |
5.0 |
16.7 |
|
Progressive disease |
15.0 |
5.6 |
|
Death |
0 |
16.7 |
|
Other |
10.0 |
0 |
|
Withdrawal by subject |
0 |
11.1 |
|
Lack of efficacy |
5.0 |
0 |
|
Non-compliance with study drug |
5.0 |
0 |
The incidence of adverse events (AEs) within the study was consistent with the established safety profile of ruxolitinib. Thrombocytopenia and anemia were the most common AEs in both Stratum 1 (50% and 55%, respectively) and Stratum 2 (78% and 44%, respectively). No new or unexpected safety events were identified. Common AEs experienced in more than 25% of patients in either strata are shown in Table 3.
Table 3. All grade and ≥Grade 3 adverse events reported in ≥25% of patients in either strata*
|
*Adapted from Guglielmelli, et al.3 |
||||
|
Adverse event, % |
Stratum 1 |
Stratum 2 |
||
|---|---|---|---|---|
|
All grades |
Grade >3 |
All grades |
Grade >3 |
|
|
Anemia |
55.0 |
25.0 |
44.4 |
16.7 |
|
Thrombocytopenia |
50.0 |
40.0 |
77.8 |
77.8 |
|
Diarrhea |
30.0 |
5.0 |
27.8 |
0 |
|
Pyrexia |
30.0 |
0 |
22.2 |
5.6 |
|
Ecchymosis |
30.0 |
0 |
11.1 |
0 |
|
Decreased platelet count |
30.0 |
25.0 |
5.6 |
5.6 |
|
Abdominal pain |
25.0 |
0 |
22.2 |
0 |
|
Decreased white cell count |
25.0 |
10.0 |
5.6 |
0 |
|
Epistaxis |
25.0 |
0 |
0 |
0 |
|
Nasopharyngitis |
20.0 |
0 |
27.8 |
0 |
|
Asthenia |
15.0 |
5.0 |
27.8 |
11.1 |
|
Cough |
10.0 |
0 |
33.3 |
0 |
|
Hypocalcemia |
10.0 |
0 |
27.8 |
0 |
Four deaths were reported whilst on treatment: 2 in Stratum 1 (cardiac arrest, n = 1; and acute myeloid leukemia, n = 1) and 2 in Stratum 2 (both multi-organ failure). Only one death was investigated as related to ruxolitinib therapy (patient with cardiac arrest).
Conclusion
Clinical responses to ruxolitinib are dose dependent and patients with low platelet counts could be underdosed and receive suboptimal therapy. Starting on lower doses at baseline due to disease-related thrombocytopenia could compromise long-term survival and outcomes. The EXPAND study demonstrated that patients with a baseline platelet count of 50–99 × 109/L can tolerate a starting dose of 10 mg ruxolitinib, as opposed to 5 mg, achieving good splenic responses without significant AEs and with significant improvements in patient symptoms.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




