All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Fedratinib in myelofibrosis
Fedratinib is a pyrimidine-based Janus kinase 2 (JAK2) inhibitor currently licenced by the U.S. Food and Drug Administration (FDA) for the treatment of adults with intermediate-2 or high-risk primary or secondary myelofibrosis (MF). It has received orphan drug designation by the European Medicines Agency (EMA) and is currently being assessed for marketing authorization in Europe. Along with ruxolitinib (JAK1/2 inhibitor), they are the only licenced JAK2 inhibitors for patients with MF. Prior to its recent approval in August 2019, fedratinib was placed on a 4-year FDA hold due to the suspicion of serious neurological toxicities.
We hereby review the long approval journey of fedratinib, including all the latest analyses from the pivotal JAKARTA and JAKARTA-2 trials that led to its FDA approval, the ongoing fedratinib trials, and its current clinical considerations. This article summarizes the review published in Blood Advances by Ann Mullaly and colleagues,1 and incorporates the updated JAKARTA-2 analysis published by Claire Harrison et al. in the American Journal of Hematology.2
Fedratinib approval timeline1
The identification of the activating JAK2 V617F mutation in 2005 and its pathogenic role in myeloproliferative neoplasms (MPN) ignited the development of many JAK2 inhibitors, including ruxolitinib and fedratinib. Following the promising preclinical data of fedratinib in JAK2 V617F knock-in mice, the first in-human phase I clinical trial started in 2008. In this study, fedratinib seemed to be efficacious and tolerable at a maximum dose of 680 mg in patients with intermediate- or high-risk MF (Figure 1). In 2011, the pivotal phase III JAKARTA trial opened for patients with intermediate- and high-risk naive MF, while a year later the phase II trial JAKARTA-2 recruited patients with MF who did not benefit from ruxolitinib treatment. During these two trials, eight participants who had been treated with fedratinib developed neurological symptoms indicative of the thiamine-deficiency condition of Wernicke encephalopathy (WE). For this, the FDA placed a clinical hold on fedratinib, and all clinical development ceased. This 4-year FDA suspension was lifted in 2017, following a central review decision that no evidence of fedratinib directly causing WE in the original cases existed, however risk factors such as a cachectic, malnourished state and uncontrolled gastrointestinal toxicity were identified. Currently, two phase III trials are ongoing with strict nutritional and thiamine monitoring (FREEDOM and FREEDOM-2) to assess fedratinib as first- or second-line in patients with MF (Figure 1).
Figure 1. Fedratinib approval timeline1

Pivotal JAKARTA and JAKARTA-2 trials
The JAKARTA and JAKARTA-2 trials are pivotal studies leading to fedratinib’s recent FDA approval. The study design and clinical findings of these trials are summarized below and shown in comparison in Table 2.
JAKARTA results1
The multicenter, randomized, JAKARTA phase III trial (NCT01437787) assessed fedratinib as frontline treatment for MF in 289 patients with intermediate-2 or high-risk disease based on the Dynamic International Prognostic Scoring System (DIPSS; Table 2).
Once daily, oral fedratinib at 400 mg or 500 mg doses was compared to placebo (three arms) for 24 weeks, in terms of spleen volume reduction (SVR ≥ 35%; primary objective) and MF-associated total symptom score (TSS) reduction based on the MF Symptom Assessment Form (MFSAF; ≥ 50% reduction; secondary objective).
At 24 weeks, SVR was achieved by 36% of patients in the 400 mg group, in 40% of patients in the 500 mg group and in 1% of patients receiving placebo. In terms of MF-symptom reduction, this occurred in 36% of patients in the 400 mg group, 34% of patients in the 500 mg arm and in 7% of patients receiving placebo (Table 2). The responses did not correlate with prognostic risk, MF subtype, or JAK2 V617F mutation status.
The most common hematological toxicity was anemia, while gastrointestinal (GI) adverse effects like nausea and diarrhea were the most common non-hematological toxicities reported. GI side effects were generally of low grade and responsive to supportive therapy.
During the JAKARTA trial, four patients with encephalopathy suggestive of WE were reported in the 500 mg fedratinib group, leading to the early termination of the trial.
JAKARTA-2 results1
The single-arm, open-label, non-randomized, phase II trial JAKARTA-2 investigated the efficacy of second-line fedratinib in 97 patients with DIPSS intermediate (1/2)- or high-risk MF disease, who had received ruxolitinib treatment without clinical benefit (clinician’s assessment).
Patients received oral fedratinib once daily, at a starting dose of 400 mg with a dose escalation at 600 mg if SVR < 50% by the end of Cycles 2 and 4. Treatment was planned for six 28-day cycles. The primary endpoint was SVR ≥ 35% at end of Cycle 6 and the key secondary objective was TSS reduction of ≥ 50%, similar to JAKARTA (Table 2).
Since, in the original analysis, ruxolitinib resistance/intolerance was not well defined and was determined by patient investigators, a recent reappraisal of the JAKARTA-2 results with more strict definitions for ruxolitinib resistance/intolerance (stringent criteria cohort; Table 1) was published by Harisson et al.2 Moreover, in this updated report of JAKARTA-2, an intention-to-treat (ITT) analysis was used instead of the original last-observation-carried-forward (LOCF) analysis of the per protocol population. This meant that patients missing assessments at end of Cycle 6 were considered non-responders.
The appraised definitions used for ‘ruxolitinib failure’ by Harrison et al. are shown in Table 1 below.
Table 1. Updated ruxolitinib definitions and analysis for patients in the JAKARTA-2 trial 2
|
ITT, intention-to-treat; RBC, red blood cell; SVR, spleen volume reduction *Response to ruxolitinib was defined as a ≥ 35% reduction in spleen volume from baseline, or a ≥ 50% reduction in spleen size from baseline. |
|
|
|
(N = 97) |
Stringent criteria cohort (n = 79) |
|
|---|---|---|
|
Ruxolitinib resistant or intolerant per investigator assessment:
Resistant: lack of response, evidence of disease progression, or loss of response to ruxolitinib following ≥ 14 days of treatment
Intolerant: discontinuation due to unacceptable toxicity after any duration of ruxolitinib exposure |
Relapsed: ruxolitinib treatment for ≥ 3 months with spleen regrowth, defined as < 10% SVR or < 30% decrease in spleen size from baseline, following an initial response* |
|
|
Refractory: ruxolitinib treatment for ≥ 3 months with< 10% SVR or < 30% decrease in spleen size from baseline |
||
|
Intolerant: ruxolitinib treatment for ≥ 28 days complicated by development of RBC transfusion requirement (≥ 2 units per month for 2 months); or Grade ≥ 3 thrombocytopenia, anemia, hematoma, and/or hemorrhage while receiving ruxolitinib |
||
Based on the above definitions and the updated ITT analysis, the results showed that the primary objective of SVR ≥ 35% was achieved by 31% of patients receiving fedratinib in the ITT population and 30% of patients in the Stringent criteria cohort. Nevertheless, all patients in the stringent criteria cohort and all but one patient in the ITT population had some degree of spleen volume reduction. With regards to symptom reduction, an MFSAF-TSS reduction of ≥ 50% was achieved by 27% of patients in the ITT population and stringent criteria cohort (Table 2). Median duration of SVR response was not reached. Eighty-two patients with data available at baseline and end of Cycle 6 of fedratinib treatment reported some decrease in symptom severity.
In terms of safety, the updated analysis of JAKARTA-2 reported similar toxicities to JAKARTA with Grade 3-4 anemias (38%), thrombocytopenias (22%), and non-hematological low grade GI toxicities, including diarrhea (58%) and nausea (56%). No cases of WE were reported in JAKARTA-2 (Table 2).
Table 2. Comparative table of study designs and findings from JAKARTA and JAKARTA-2 trials1,2
|
DIPSS, Dynamic International Prognostic Scoring System; ET, essential thrombocythemia; FEDR, fedratinib; GI, gastrointestinal; ITT, intention-to-treat; JAK, Janus kinase; MF, myelofibrosis; MFSAF, MF Symptom Assessment Form, PV, polycythemia vera; SCC, Stringent criteria cohort; SVR, spleen volume reduction; WE, Wernicke encephalopathy |
||||
|
|
JAKARTA1 |
JAKARTA-2 – updated analysis1,2 |
||
|---|---|---|---|---|
|
Study design |
Phase III, randomized, placebo-controlled |
Phase II, single arm, non-randomized |
||
|
Dosing/arms |
Placebo FEDR 400 mg FEDR 500 mg |
FEDR 400 mg |
||
|
Patient eligibility |
Primary MF (post-ET/-PV) Intermediate-2 or high DIPSS risk JAK inhibitor naive |
Primary MF (post-ET/-PV) Intermediate-1 (symptomatic), intermediate-2 or high DIPSS risk |
||
|
Patient enrollment |
N = 289 |
N = 97 |
||
|
Primary endpoint |
≥ 35% SVR |
≥ 35% SVR |
||
|
Key secondary endpoints |
≥ 50% total MF symptom reduction based on MFSAF |
≥ 50% total MF symptom reduction based on MFSAF |
||
|
Originally published response rates |
FEDR 400 mg |
FEDR 500 mg |
Placebo |
FEDR 400 mg |
|
SVR (≥ 35% reduction) |
36% |
40% |
1% |
ITT population: 31% (n = 97) SCC population: 30% (n = 79) |
|
MF-related total symptom score reduction (≥ 50% reduction) |
36% |
34% |
7% |
ITT population: 27% (n = 90) SCC population: 27% (n = 74) |
|
Toxicity |
Grade 1-2 GI toxicities Grade 3-4 cytopenias Suspected WE (n = 4 in 500 mg FEDR arm) |
Consistent with JAKARTA low grade GI toxicities and Grade 3-4 thrombocytopenia |
||
Ongoing trials & current clinical considerations
Two phase III trials, FREEDOM (NCT03755518) and FREEDOM-2 (NCT03952039), investigating the efficacy and safety of fedratinib in patients with intermediate/high-risk MF as frontline or second line treatment are ongoing. The primary endpoint of both trials is the proportion of patients achieving an SVR ≥ 35%.1,2
Both trials include risk‐mitigation strategies for WE and GI toxicities. These involve strict routine monitoring of thiamine levels and thiamine supplementation as needed, as well as proactive management of GI events with the use of antiemetics and antidiarrheals.
Current clinical considerations when using fedratinib are shown in Figure 2. To date, many unanswered questions exist concerning the management of patients with MF needing second-line treatment. Concerns exist when switching patients from ruxolitinib to fedratinib, due to potential deterioration after JAK inhibitor withdrawal. Current practices involve the immediate switch from one drug to the other without washing off, while tapering and then switching is also recommended (Figure 2). With the recent approval of fedratinib, another unresolved issue exists: how will clinicians choose between fedratinib or ruxolitinib as frontline therapy for patients with intermediate/high-risk MF? Until the initiation of large comparative trials between ruxolitinib and fedratinib in this MF patient population, this will remain unanswered.
Figure 2. Current clinical considerations for fedratinib treatment 1
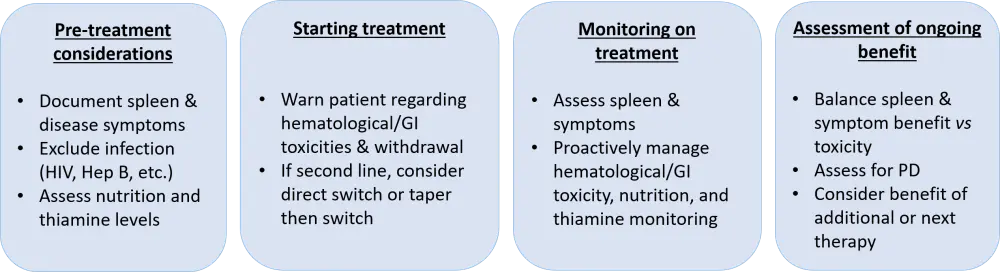
GI, gastrointestinal; PD, progressive disease
Conclusion
From the pivotal JAKARTA-1/-2 trials and the updated stringent analysis of JAKARTA-2, it is evident that patients with advanced MF who have been substantially pre-treated with ruxolitinib can achieve strong responses with fedratinib. With the approval and re-evaluation of the pivotal trials, it seems that the initially reported encephalopathies were not related to fedratinib treatment. Nevertheless, two ongoing phase III trials (FREEDOM-1/-2) that are re-assessing the efficacy and safety of fedratinib in the same patient populations are using stringent risk‐mitigation strategies, for both WE and the common fedratinib-related adverse effect of gastrointestinal toxicity. The results of the ongoing FREEDOM trials, together with real-world data, are anticipated to improve the treatment algorithm and management of MF.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

