All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
FREEDOM trial: Primary analysis
Fedratinib, an oral Janus kinase 2 (JAK2) inhibitor previously reviewed on the MPN Hub, is currently under investigation for the treatment of patients diagnosed with myelofibrosis (MF) and with prior ruxolitinib exposure.1 Long-term data regarding fedratinib in the setting are currently limited due to early treatment termination.1
In order to address this, Gupta et al.1 recently published a preliminary analysis from the single-arm phase IIIb FREEDOM trial (NCT03755518) investigating the safety and efficacy of fedratinib in patients with MF previously treated with ruxolitinib. The authors also discussed strategies to mitigate gastrointestinal adverse events (AE), thiamine level reductions, and potential encephalopathy.1 We summarize the key findings in the article below. For more information on how to manage AEs associated with fedratinib, watch our previous interview with Ruben Mesa.
Study design
- Key eligibility criteria:
- Confirmed primary MF diagnosis, post-polycythemia vera MF, or post-essential thrombocythemia MF with ≥3 months prior ruxolitinib treatment
- Eastern Cooperative Oncology Group performance status (ECOG PS) ≤2
- Intermediate or high-risk
- Baseline platelet count ≥50 x 109/L
- Fedratinib 400 mg once daily in 28-day cycles
- Strategies for AE mitigation:
- Antiemetics
- Antidiarrheals
- Thiamine supplementation
- Surveillance for potential encephalopathy
Results
- A total of 38 patients were included in the analysis.
- Baseline patient characteristics are shown in Table 1.
Table 1. Baseline patient characteristics*
|
DIPSS, Dynamic International Prognostic Scoring System; ET, essential thrombocythemia; MF, myelofibrosis; *Data from Gupta, et al.1 |
|
|
Characteristic, % (unless otherwise stated) |
All patients |
|---|---|
|
Median age, years |
68.5 |
|
Median treatment duration, weeks |
38 |
|
Diagnosis |
|
|
PMF |
60.5 |
|
Post-PV MF |
23.7 |
|
Post-ET MF |
15.8 |
|
DIPSS classification |
|
|
Intermediate-1 |
34.2 |
|
Intermediate-2 |
47.4 |
|
High risk |
18.4 |
|
Median spleen volume, mL |
1,831.6 |
- At the end of Cycle 6, 25.7 patients achieved ≥35% spleen volume reduction (SVR)
- 62.9% of patients achieved a best overall response of ≥35% SVR.
- Of the patients who achieved ≥35% SVR, 86.4% maintained a response at cutoff.
- At the end of Cycle 3, 58.3% of patients achieved ≥50% reduction in total symptom score
- At the end of Cycle 6, 44.4% of patients achieved ≥50% reduction in total symptom score.
- A total of 89.5% of patients had one treatment-emergent AE; 7.9% of which were serious.
Common AEs are shown in Figure 1.
Figure 1. Common adverse events*
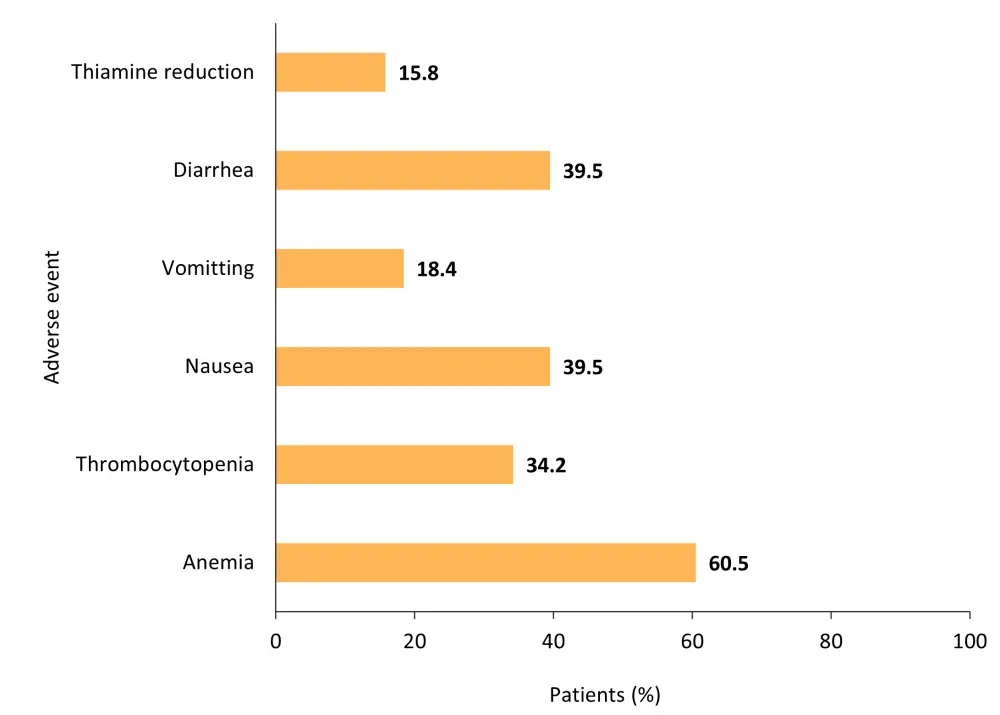
*Data from Gupta, et al.1
- Most gastrointestinal AEs were of Grade 1 or 2 severity.
- Reduction in thiamine levels were also of Grade 1 or 2 severity.
- There were no treatment discontinuations due to thiamine levels.
- A total of two deaths occurred during treatment and seven occurred during follow-up.
- None of the deaths were deemed to be study related.
Conclusion
Primary analysis from the FREEDOM study highlights durable spleen and symptom responses as well as SVR. Gastrointestinal AEs were easily mitigated, and no cases of encephalopathy were reported. Although the sample size for the analysis was small, the overall safety and efficacy data collected support the use of fedratinib in patients with MF and prior ruxolitinib exposure.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



