All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
FREEDOM2: Efficacy and safety of fedratinib vs BAT in relapsed/refractory MF
Do you know... What is the median time to treatment discontinuation in patients with myelofibrosis treated with ruxolitinib?
Fedratinib, a Janus kinase 2 inhibitor (JAK2i), was approved by the U.S. Food and Drug Administration (FDA) in 2021 for the use in patients with disease-related splenomegaly or symptoms in adult patients with primary myelofibrosis (MF), or secondary MF, i.e., post-polycythemia vera/post-essential thrombocytosis MF who are treatment-naïve or had received prior ruxolitinib therapy.1 Ruxolitinib remains a recommended treatment option for first-line treatment of MF-associated splenomegaly.1,2 However, almost half of patients discontinue ruxolitinib treatment after 3–5 years. Fedratinib has been investigated as a second-line treatment option for patients who discontinue ruxolitinib treatment to address this unmet need.2
The MPN Hub is pleased to summarize the rationale for and latest data from FREEDOM2 investigating fedratinib after ruxolitinib therapy for the treatment of MF.
FREEDOM21
- FREEDOM2 (NCT03952039) is a phase III, randomized trial which investigated the safety and efficacy of fedratinib vs best available therapy (BAT) in patients with MF previously treated with ruxolitinib.
- Eligible patients (N = 201) were randomized 2:1 to receive fedratinib (400 mg/day; n = 134)) or BAT (n = 67) (Figure 1).
Figure 1. FREEDOM2 study design*
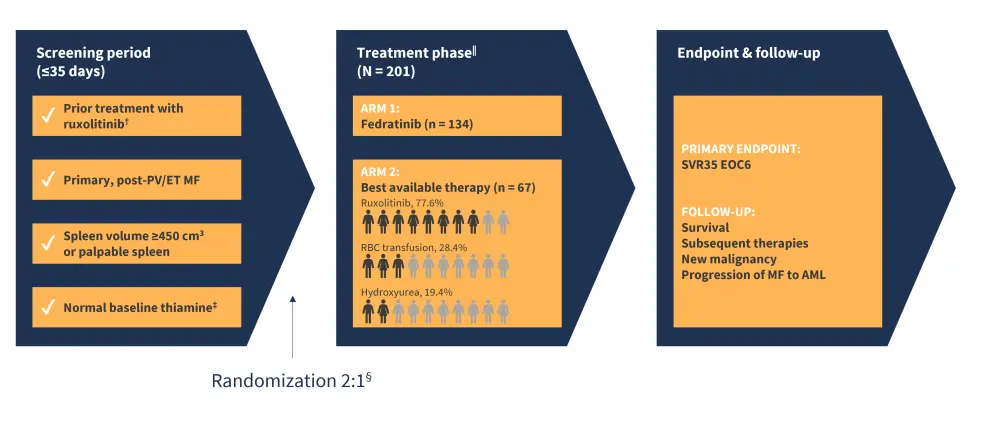
AML, acute myeloid leukemia; EOC6, end of cycle six; ET, essential thrombocytopenia; MF, myelofibrosis; PV, polycythemia vera; SVR35, spleen reduction volume ≥35%.
*Adapted from Harrison, et al.1
†Patients must have received ruxolitinib for ≥3 months with <10% SVR by MRI or <30% decrease from baseline in spleen size by palpation or regrowth to relapsed/refractory disease, or for ≥28 days with development of RBC transfusion requirement or Grade ≥3 thrombocytopenia, anemia, hematoma, or hemorrhage.
‡Thiamine normal baseline considered 70–180 nmol/L.
§Stratification factors by spleen size by palpation, platelet counts, and ruxolitinib relapsed/refractory vs intolerant.
‖Crossover fedratinib therapy permitted: before cycle six for confirmed disease progression, and after cycle six response assessment.
Patient population1
- At baseline, the median age was 70 years across cohorts.
- Overall, 91% of patients had JAK2, CALR, or MPL mutations, of which:
- JAK2, 70.6%;
- CALR, 19.4%; and
- MPL, 2.5%.
- Constitutional symptoms were present in 62.7% of patients.
- Gastrointestinal (GI) mitigation strategy, including thiamine monitoring and guidelines for supplementation, were assessed proactively throughout.
Efficacy1
- The primary endpoint was met by more patients treated with fedratinib (35.8%) compared with those receiving BAT (6.0%) (Figure 2).
- Patients treated with fedratinib also experienced improved rates of symptom response, and SVR35 benefit compared with BAT across all clinically relevant subgroups (Figure 2).
Figure 2. FREEDOM2 key efficacy outcomes *
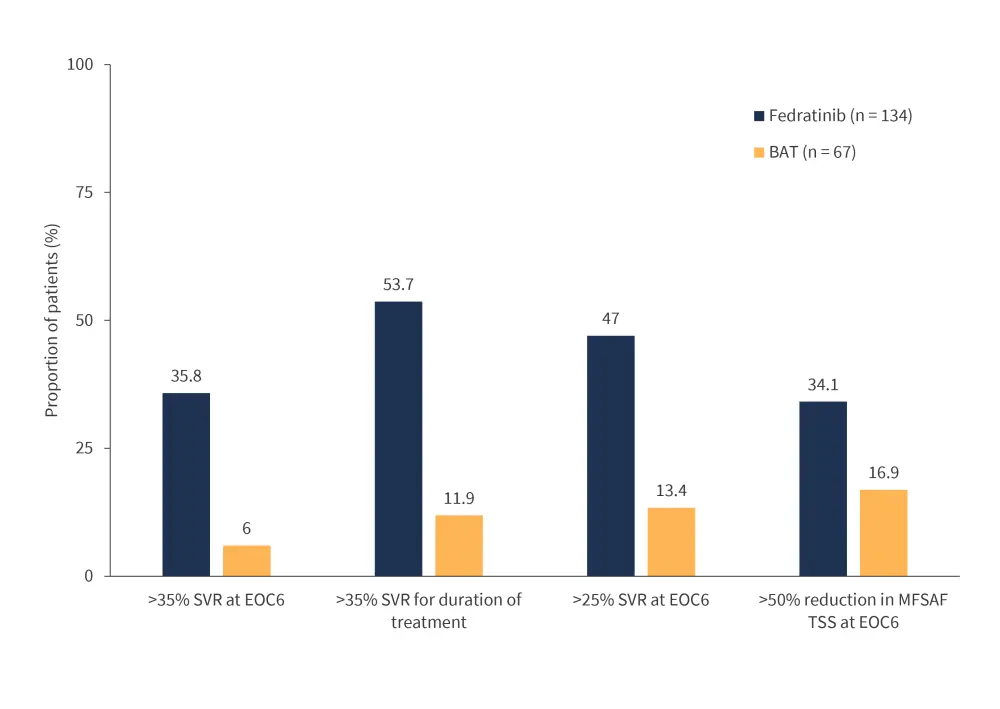
BAT, best available treatment; EOC6, end of cycle six; MFSAF TSS, Myelofibrosis Symptom Assessment Form Total Symptom Score; SVR, spleen volume reduction.
*Adapted from Harrison, et al.1
- At data cutoff, median treatment duration was higher in the fedratinib arm vs BAT (43 weeks vs 24.7 weeks).
- 74.6% of patients in the fedratinib arm completed six cycles of treatment;
- 46 patients in the BAT arm crossed over to receive fedratinib.
- Treatment discontinuation data are outlined in Figure 3.
Figure 3. Treatment discontinuation vs treatment ongoing at data cutoff, and reasons for discontinuation.*
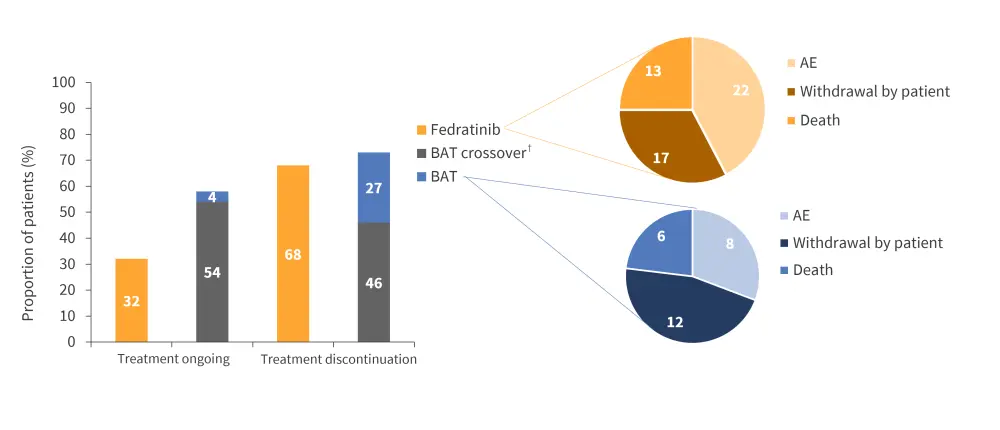
AE, adverse event; BAT, best available treatment.
*Adapted from Harrison, et al.1
†BAT crossover includes patients who were assigned to the BAT treatment arm but crossed over to fedratinib treatment.
- In a subgroup analysis:
- There was a trend towards a higher proportion of patients achieving the primary endpoint who were intolerant to prior ruxolitinib treatment compared with patients who were relapsed/refractory (56.5% vs 31.5%).
- Patients who had a lower platelet count (50–100 × 109/L) at baseline also achieved higher SVR35 compared with a higher platelet count (≥100 × 109/L) (47.1% vs 35.3%).
Safety1
- Overall, Grade 3/4 treatment-related-adverse events (TRAEs) were higher in patients receiving fedratinib vs BAT (38.8% vs 11.9%).
- The most frequently reported TRAE in both arms were blood and lymphatic system disorders, including thrombocytopenia (11.9% vs 3%) and anemia (9% vs 9%) (Figure 4).
Figure 4. Grade 3/4 TRAEs during the first six treatment cycles in ≥5% of patients in any group*
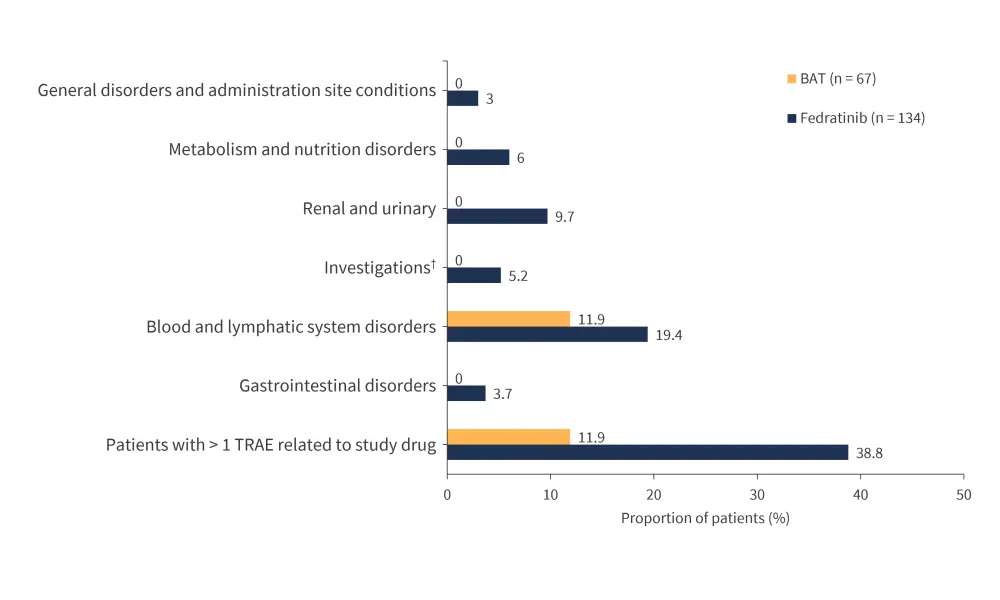
BAT, best available treatment; TRAE, treatment-related adverse event.
*Adapted from Harrison, et al.1
†Including thiamine decreased, alanine aminotransferase increased, and vitamin B1 decreased.
- GI AEs were reported less frequently than in the JAKARTA2 trial.
- Low thiamine was reported more often in patients treated with fedratinib compared with BAT.
- Wernicke’s encephalopathy was reported once and controlled with thiamine supplementation.
- Treatment interruption or dose reduction was higher in patients receiving fedratinib vs BAT (52.2% vs 29.9%).
- Permanent discontinuation was also higher in patients receiving fedratinib vs BAT (9.7% vs 6%).
- In total, seven deaths were reported (Table 1).
Table 1. Causes of mortality in patients treated with fedratinib compared with BAT*
|
BAT, best available treatment; MF, myelofibrosis; TEAE, treatment-emergent adverse event. |
||
|
Cause of mortality, n |
Fedratinib (n = 134) |
BAT (n = 67) |
|---|---|---|
|
All causes |
6 |
1 |
|
MF disease progression |
2 |
0 |
|
Sepsis from rapid onset of splenomegaly |
1 |
0 |
|
COVID-19 |
2 |
1 |
|
TEAE of acute kidney injury related to treatment |
1 |
0 |
Conclusion1
Data from the FREEDOM2 trial continue to support fedratinib use after ruxolitinib failure in patients with MF. SVR25 and SVR35 at any time, especially after six cycles, was achieved by more patients receiving fedratinib compared with BAT. This improvement was observed in all subgroups, including patients with a lower platelet count (50–100 × 109/L) at baseline.
No new safety data were identified, with GI AEs mostly Grade 1/2, consistent with other studies such as FREEDOM and JAKARTA2. Importantly, the majority (77.6%) of patients receiving BAT received ruxolitinib rechallenge; demonstrating a clear clinical need for an alternative treatment post ruxolitinib treatment.
Overall, findings from FREEDOM2 support the use of fedratinib as a promising treatment for second-line treatment of MF.
This educational resource is independently supported by Bristol Myers Squibb. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




