All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Defining ruxolitinib failure and transition to next-line therapy in MF
Your opinion matters
How would you define myelofibrosis treatment failure? A. A lack of spleen reduction B. A lack of symptom reduction C. A lack of either spleen or symptom reduction D. Symptom relief deemed inadequate by the patient
Your opinion matters
How would you identify that patients are no longer responding to MF first-line treatment? A. Plateau in response B. Loss of response, defined by symptoms worsening or returning to baseline C. Symptom relief deemed inadequate by the patient D. More than one of the above/other
Your opinion matters
How would you switch patients from a failed first-line MF treatment to second-line treatment? A. Tapering treatment and wait B. Introduce second-line therapy once ruxolitinib has been discontinued C. Overlap ruxolitinib tapering with second-line therapy introduction D. Other
Ruxolitinib is a Janus kinase 1/2 (JAK1/JAK2) inhibitor commonly used as a first-line therapy for the treatment of myelofibrosis.1 However, 50–72% of patients experience a loss of response or intolerability to ruxolitinib within 3–5 years of treatment, and discontinuation is complicated by serious adverse events risks and poor outcomes. There is currently a lack of consensus on the definition of ruxolitinib failure and guidance on the transition to next-line therapy.1
A group of myelofibrosis experts, including steering committee member John Mascarenhas, formed a Delphi panel to lay out the criteria and guidance for the management of these patients.1 The MPN Hub is pleased to summarize this consensus here.
Determining therapy failure1
Ruxolitinib failure can be considered in three scenarios:
- Non-response/primary refractory status
- A loss of response
- Progressive disease status
Primary refractory consensus
A 3-month period of a maximum tolerated dose of ruxolitinib is required before primary refractory status can be determined. Following this period, spleen and symptom response can be taken for comparison with baseline. Figure 1 outlines the consensus criteria for primary refractory status.
Figure 1. Consensus criteria for primary refractory status*
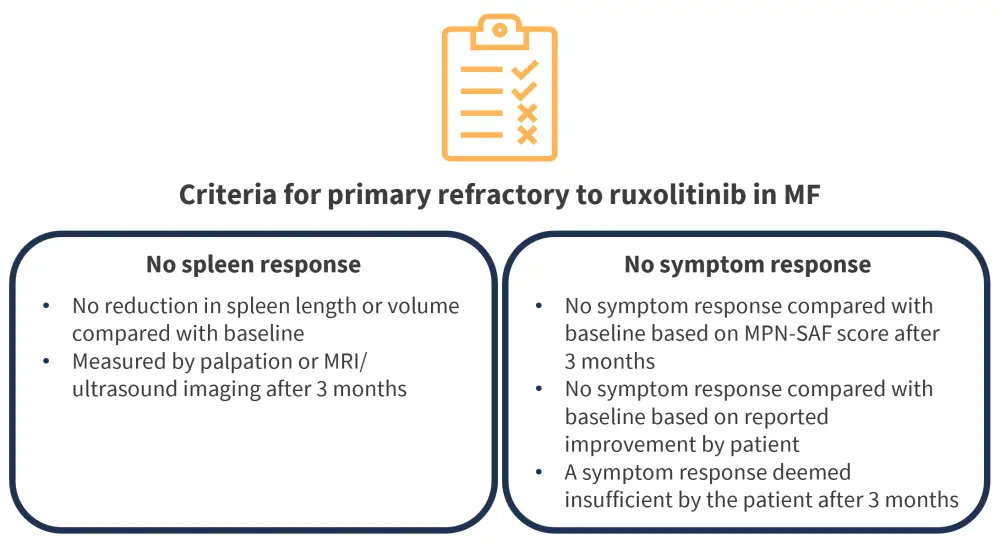
LCM, Left costal margin; MF, myelofibrosis; MPN-SAF, Myeloproliferative Neoplasm Symptom Assessment Form; MRI, magnetic resonance imaging.
*Data from Mascarenhas, et al.1
Loss of response consensus
A loss of response to ruxolitinib after an initial clinical response can be established by measuring changes in the same two clinical features; spleen and symptom response. These measures are considered in comparison with either baseline or best achieved response during the initial response period. Symptom changes can be measured using the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) or using the patient-reported experiences. Criteria and assessments for a loss of response are outlined in Figure 2.
Figure 2. Consensus criteria for loss of response to ruxolitinib*
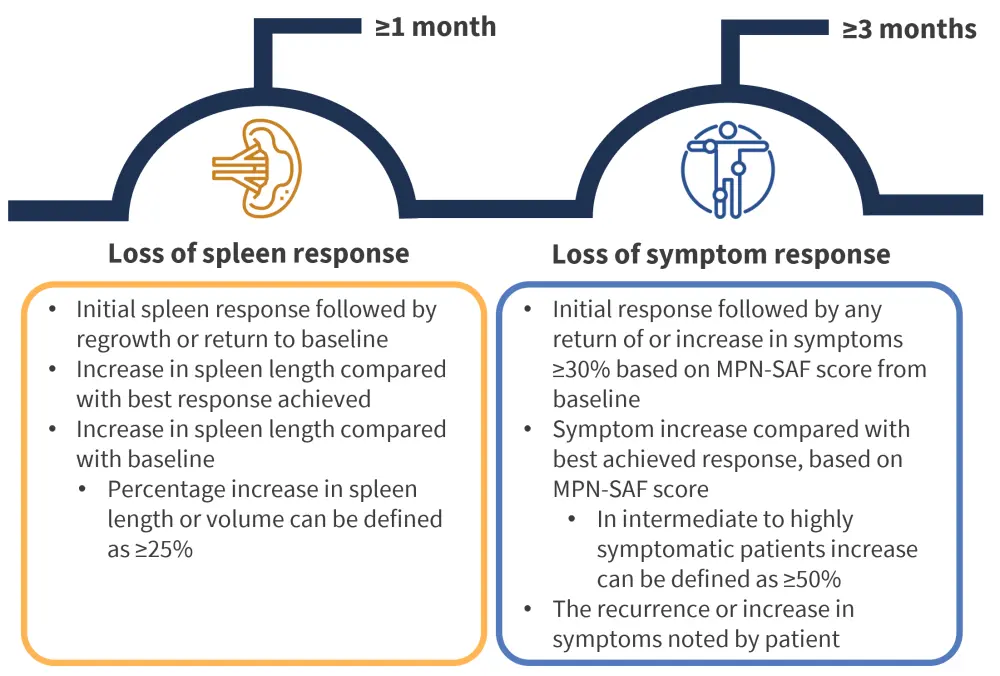
MPN-SAF, Myeloproliferative Neoplasm Symptom Assessment Form.
*Data from Mascarenhas, et al.1
Progressive disease consensus
Ruxolitinib failure is also considered in patients with characteristics of progressive disease. These characteristics are outlined in Figure 3. Progression of splenomegaly requires treatment adaption due to its links with splenic extramedullary hematopoiesis, hypertension, and increased cytopenia.
Figure 3. Consensus criteria for progressive disease status*
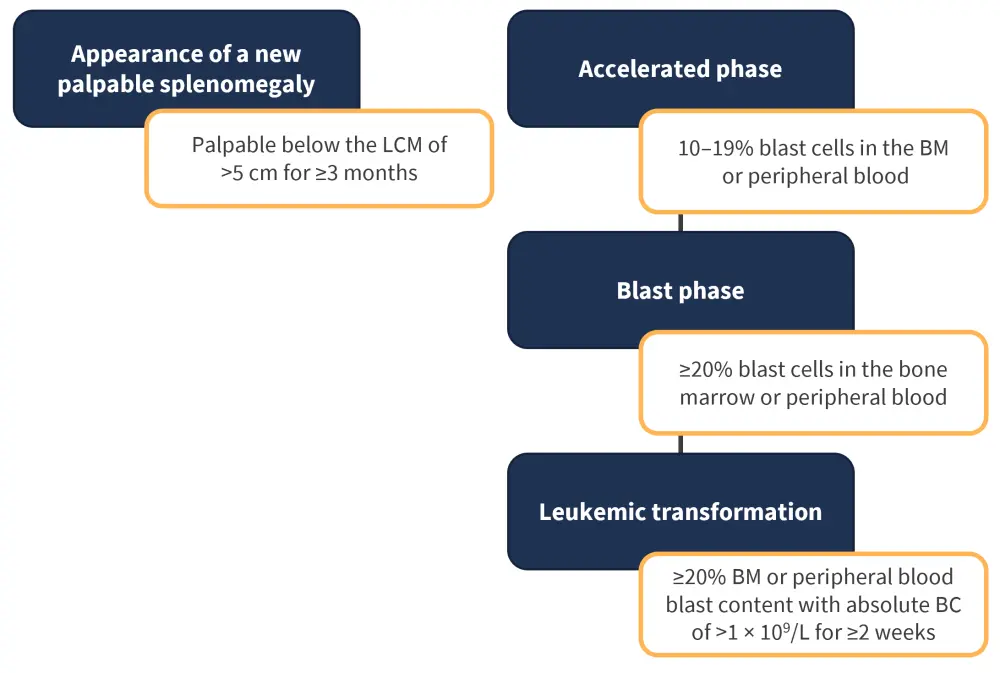
BC, blast count; BM, bone marrow; LCM, Left costal margin.
*Data from Mascarenhas, et al.1
Transition to next-line therapy1
The transition to next-line therapy presents challenges, namely the risk of ruxolitinib discontinuation syndrome (RDS); characterized by relapse of symptoms, accelerated splenomegaly, and worsening of cytopenias. Figure 4 lists strategies for the transition to next-line therapy to mitigate the risk of RDS.
Figure 4. Strategies for next-line therapy transition*

*Data from Mascarenhas, et al.1
Conclusion
The lack of clear clinical guidelines on the definition of first-line ruxolitinib failure presents a challenge to transitioning patients to further lines of therapy. This Delphi panel reached a consensus on determining ruxolitinib failure based on non-response, loss of response, and characteristics of progressive disease. These criteria may serve to inform clinical decision making in the absence of evidence-based guidance. Strategies to limit the risk of adverse events and RDS are also defined to guide the transition of therapy based on individual risk factors and comorbidities.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content





