All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Long-term survival and safety outcomes with momelotinib
Momelotinib is a potent inhibitor of Janus kinase 1 and 2 (JAK1/2) and activin A receptor type I (ACVR1), also known as activin receptor-like kinase-2 (ALK-2), and is currently under evaluation for approval by the U.S. Food and Drug Administration (FDA) for the treatment of patients with myelofibrosis (MF) and anemia.1
To date, results from phase III studies have demonstrated that momelotinib leads to reduced severity of anemia and subsequent transfusion independence (TI) relative to ruxolitinib, and is associated with an increased splenic response.
In Leukemia, Ruben Mesa, et al.,2 recently published longer-term overall survival (OS), leukemia-free survival (LFS), and safety data following extended momelotinib treatment of patients with MF in the phase III SIMPLIFY-1 (NCT01969838) and SIMPLIFY-2 (NCT02101268) trials, including those enrolled in an ongoing open-label, extended access clinical trial (NCT03441113).2 We are pleased to provide a summary of this publication here.
Study design
The SIMPLIFY-1 and SIMPLIFY-2 trials compared momelotinib to ruxolitinib in JAK inhibitor-naïve patients with MF and to ruxolitinib/best available therapy (BAT) in the relapsed/refractory MF setting, respectively. The study designs of these two trials can be found here. In both trials, patients who were initially randomized to ruxolitinib or ruxolitinib/BAT could cross over to momelotinib at Week 24. Patients who tolerated momelotinib and who did not have disease progression during the SIMPLIFY trials could continue momelotinib in the open-label extended access trial.
The analysis by Mesa, et al. included patients from the three trials to investigate the following parameters:
- OS
- LFS
- Mean daily dosing
- Association between baseline patient characteristics and survival outcomes
- Association between well-established efficacy endpoints and OS
- Long-term safety data
- Red blood cell TI rate at 24 weeks and overall (a TI responder was defined as a patient whose hemoglobin was ≥8 g/dL between Weeks 12 and 24)
- Splenic volume reduction >35% at Week 24 and total symptom scores
Results
Long-term survival data
In the SIMPLIFY-1 trial, the median follow-up was 3.43 years and 3.47 years in the momelotinib arm and ruxolitinib (crossover to momelotinib) arm, respectively:
- Death: 30.8% vs 33.8%
- Leukemic transformation: 5.6% vs 4.2%
- OS and LFS curves were similar between the two arms
- Median OS and LFS were not reached in both arms and the 5-year survival probability was 55%
In the SIMPLIFY-2 trial, the median follow-up was 3.07 years and 3.22 years in the momelotinib arm and ruxolitinib/BAT (crossover to momelotinib) arm, respectively:
- Death: 45.2% vs 44.2%.
- Leukemic transformation: 6.7% vs 1.9%.
- Median OS: 2.9 years vs 3.1 years (hazard ratio, [HR], 0.98; 95% confidence interval [CI], 0.59–1.62)
- Median LFS: 2.8 years vs 3.1 years (HR, 0.97; 95% CI, 0.59–1.60)
The 2-year OS and LFS were similar for both studies (Figure 1).
Figure 1. 2-year OS and LFS in A SIMPLIFY-1 and B SIMPLIFY-2*
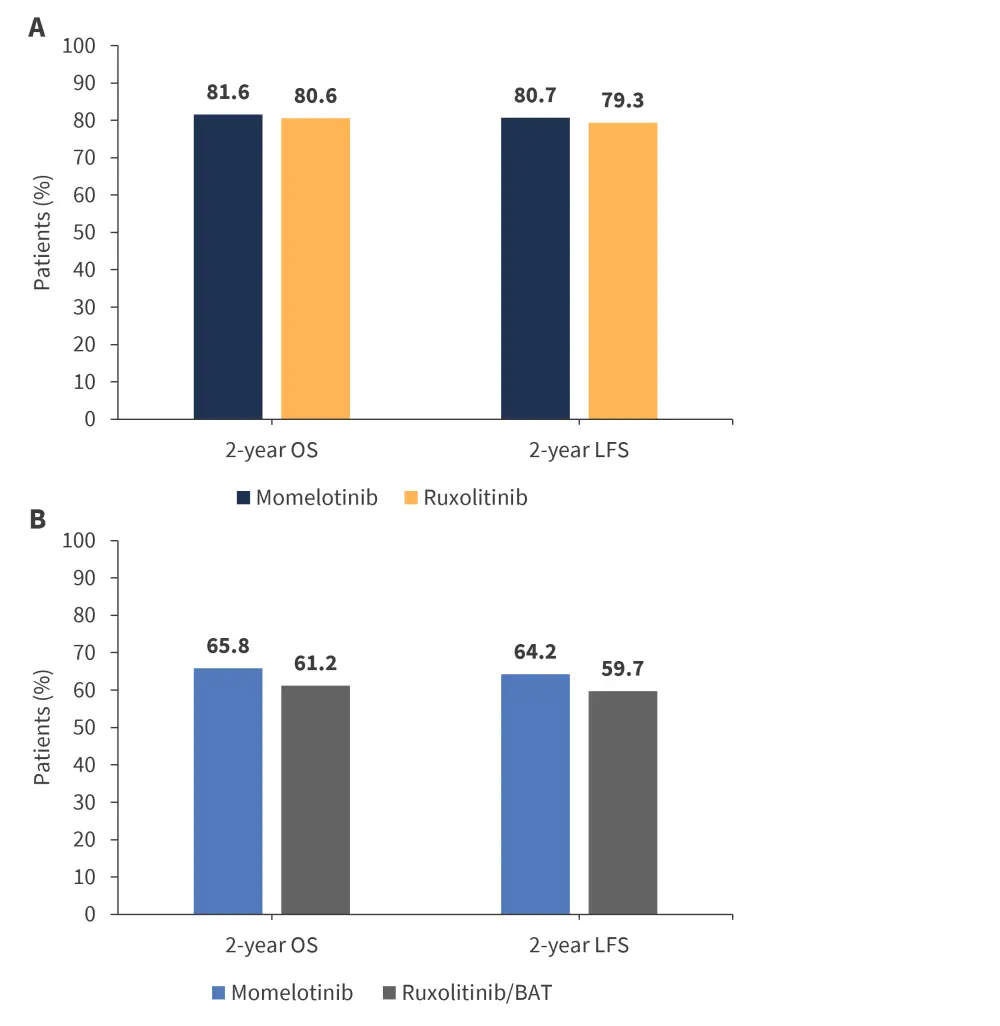
BAT, best available therapy; LFS, leukemia-free survival; OS, overall survival.
*Adapted from Mesa, et al.2
Association between patient characteristics and survival
The baseline patient characteristics found to be associated with OS are shown in Table 1 (univariate analysis) and Table 2 (multivariate analysis).
- In the SIMPLIFY-1 trial, the characteristics associated with improved OS were TI status, higher hemoglobin, and higher platelet levels, whereas the characteristics associated with a poorer OS were increased spleen volume, higher white blood cell counts, and IPSS-high and -intermediate-2 risk disease.
- The multivariate analysis demonstrated that IPSS risk status, white blood cell count, and TI status at baseline had independent prognostic value for OS.
- Similar associations were observed in the univariate analysis of the SIMPLIFY-2 trial.
- The multivariate analysis showed that TI status, hemoglobin levels, spleen volume, and white blood cell count had independent prognostic value for OS.
- The MPN subtype (primary MF or secondary MF) had no prognostic value for OS in both studies.
Table 1. Univariate analysis of baseline characteristics in the SIMPLIFY-1 and SIMPLIFY-2 trials*
|
CI, confidence interval; DIPSS, Dynamic International Prognostic Scoring System; Hb, hemoglobin; Int-2, intermediate-2; IPSS, International Prognostic Scoring System; NR, not reached; OS, overall survival; TI, transfusion independence; WBC, white blood cells. |
|||||
|
Characteristic |
Prevalence, |
Median OS, |
2-year OS, |
HR (95% CI) |
p value |
|---|---|---|---|---|---|
|
SIMPLIFY-1 (n = 432) |
|||||
|
TI |
24.9 |
NR |
86.0 |
0.373 |
<0.0001 |
|
Hb, ≥10 g/dL |
27.3 |
NR |
83.3 |
0.351 |
<0.0001 |
|
Spleen volume, >2,000 cm3 |
35.6 |
NR |
74.2 |
1.448 |
0.0288 |
|
Platelets, >200 × 109/L |
28.1 |
NR |
84.5 |
0.466 |
0.0035 |
|
IPSS, high risk |
44.0 |
3.76 |
72.2 |
5.494 |
<0.0001 |
|
IPSS, Int-2 |
28.0 |
NR |
85.7 |
2.792 |
<0.0001 |
|
WBC, ≥10 × 109/L |
36.1 |
5.31 |
76.4 |
1.466 |
0.0258 |
|
SIMPLIFY-2 (n = 156) |
|||||
|
TI |
23.5 |
NR |
80.7 |
0.319 |
0.0002 |
|
Hb, ≥10 g/dL |
35.3 |
NR |
67.9 |
0.335 |
0.0003 |
|
Spleen volume, >2,000 cm3 |
54.8 |
2.19 |
53.2 |
2.329 |
0.0006 |
|
DIPSS, high-risk |
70.4 |
1.18 |
24.0 |
5.043 |
<0.0001 |
|
DIPSS, Int-2 |
44.4 |
5.31 |
70.9 |
1.651 |
<0.0001 |
|
WBC, ≥10 × 109/L |
62.7 |
1.33 |
38.4 |
3.335 |
<0.0001 |
Table 2. Multivariate analysis of baseline characteristics from the SIMPLIFY trials*
|
CI, confidence interval; Hb, hemoglobin; Int, intermediate; IPSS, International Prognostic Scoring System; TI, transfusion independence; WBC, white blood cells. |
||
|
Characteristic |
Hazard ratio (95% CI) |
p value |
|---|---|---|
|
SIMPLIFY-1 (n = 432) |
||
|
IPSS risk |
||
|
High vs Int-1 |
4.293 (2.160–8.532) |
<0.0001 |
|
Int-2 vs Int-1 |
2.759 (1.373–5.546) |
0.0044 |
|
TI vs non-TI |
0.474 (0.325–0.691) |
0.0001 |
|
WBC, ≥10 × 109/L vs <10 × 109/L |
1.648 (1.160–2.342) |
0.0054 |
|
SIMPLIFY-2 (n = 156) |
||
|
TI vs non-TI |
0.226 (0.097–0.524) |
0.0005 |
|
Spleen volume, >2,000 cm3 vs ≤2,000 cm3 |
1.905 (1.128–3.216) |
0.0159 |
|
Hb, 8 g/dL to <10 g/dL vs <8 g/dL |
0.427 (0.239–0.762) |
0.0040 |
|
WBC, ≥10 × 109/L vs <10 × 109/L |
4.498 (2.655–7.621) |
<0.0001 |
Association between clinical endpoints and survival
In the SIMPLIFY-1 trial, TI responders at Week 24 had significantly improved OS compared with TI non-responders in the momelotinib arm (3-year OS: 77.2% vs 51.6%; HR, 0.323; p < 0.0001), which was then confirmed in the multivariate analysis (HR, 0.311; 95% CI, 0.173–0.559; p < 0.0001). The difference between TI responders and TI non-responders in the SIMPLIFY-2 trial was not statistically significant (2-year OS: 66.1% vs 57.0%; p = 0.4193) in the momelotinib arm. In the ruxolitinib (SIMPLIFY-1) and ruxolitinib/BAT (SIMPLIFY-2) crossover arms, there was a trend towards improved OS; however, it was not statistically significant (p = 0.0954 and p = 0.2326, respectively).
In the context of total symptom score, there were no significant differences between arms in both studies. In the SIMPLIFY-1 trial, patients who achieved splenic volume reduction response at Week 24 had significantly better OS compared with non-responders in the ruxolitinib arm (p = 0.0078); the improvement in OS was significant in the momelotinib arm.
Longer-term safety data
The median duration of momelotinib treatment in the SIMPLIFY-1 and SIMPLIFY-2 trials was 17.7 months and 9.2 months, respectively. The safety profile of momelotinib was similar to ruxolitinib during the randomized treatment period of the SIMPLIFY-1 trial when considering treatment-emergent adverse events (TEAEs). The frequency of Grade 3/4 anemia was lower with momelotinib than ruxolitinib in the SIMPLIFY-1 trial (6.1% vs 22.7%, respectively) and the SIMPLIFY-2 trial (13.5% vs 17.3%, respectively). The prevalence of any grade TEAEs was similar in both studies across all treatment groups (Table 3).
The most common any grade TEAEs in the SIMPLIFY-1 trial were thrombocytopenia (18.7%) and anemia (25.8%) in patients receiving momelotinib, and anemia in patients receiving ruxolitinib (37.5%). The most common any grade TEAEs in the SIMPLIFY-2 trial were diarrhea (32.7%) in patients receiving momelotinib, and anemia (19.2%) and fatigue (19.2%) in patients receiving ruxolitinib/BAT.
Table 3. Most common Grade 3/4 TEAEs in each treatment group in SIMPLIFY-1 and SIMPLIFY-2*
|
BAT, best alternative treatment; MMB, momelotinib; Rux, ruxolitinib; TEAE, treatment-emergent adverse event. |
|||
|
SIMPLIFY-1 |
MMB (n = 214) |
Rux (n = 216) |
Extended duration MMB (n = 411) |
|---|---|---|---|
|
Any grade TEAEs, % |
92.5 |
95.4 |
97.3 |
|
Grade 3/4 TEAEs, %† |
34.6 |
43.5 |
64.0 |
|
Thrombocytopenia |
7.0 |
4.6 |
14.6 |
|
Anemia |
6.1 |
22.7 |
13.1 |
|
Pneumonia |
2.3 |
1.4 |
7.8 |
|
SIMPLIFY-2 |
MMB (n = 104) |
Rux/BAT (n = 52) |
Extended duration MMB (n = 144) |
|
Any grade TEAEs, % |
97.1 |
88.5 |
98.6 |
|
Grade 3/4 TEAEs, %† |
54.8 |
42.3 |
72.9 |
|
Anemia |
13.5 |
17.3 |
23.6 |
|
Thrombocytopenia |
10.6 |
5.8 |
16.0 |
Of the 118 patients who entered the momelotinib extended access protocol following the completion of SIMPLIFY-1 or SIMPLIFY-2, 88 continued treatment for more than 5 years. No new safety concerns were raised, suggesting long-term tolerance.
During the 24-week randomization period and the extended duration, the momelotinib dose intensity was maintained, whereas induced or worsening myelosuppression required lower starting doses or dose reductions in patients treated with ruxolitinib.
Conclusion
The study by Mesa, et al. reports for the first time, the longer-term survival data from the two phase III SIMPLIFY trials with extended protocol. The results suggest that JAK inhibitor-naïve patients receiving momelotinib or ruxolitinib followed by momelotinib (SIMPLIFY-1) or ruxolitinib-pretreated patients receiving momelotinib or ruxolitinib/BAT followed by momelotinib (SIMPLIFY-2) may achieve a good OS and LFS with extended momelotinib treatment, which may be attributed to improvements in anemia and TI.
The prevalence of adverse events was similar in both treatment groups, but with patients on momelotinib receiving full doses for longer periods than those on ruxolitinib, with a more favorable hematologic safety profile.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

