All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Myeloproliferative neoplasms: Disease overview
Do you know... What are the main genetic abnormalities commonly identified in patients with MPN?
Myeloproliferative neoplasms (MPN), a rare form of Philadelphia chromosome-negative hematological malignancies, represent a clonal neoplastic disorder affecting myeloid hematopoietic stem cells.1 There are three main disease subtypes (Figure 1):
- Myelofibrosis (MF), which is identifiable by bone marrow scarring and fibrosis; a small proportion of patients may also progress to the blast phase, which typically presents as acute myeloid leukemia and is refractory to conventional therapeutic options.1
- Essential thrombocythemia (ET), which is characterized by an overproliferation of platelets.1
- Polycythemia vera (PV), which is characterized by an overproduction of red blood cells.1
Figure 1. Clinical features of the different disease subtypes of MPN*
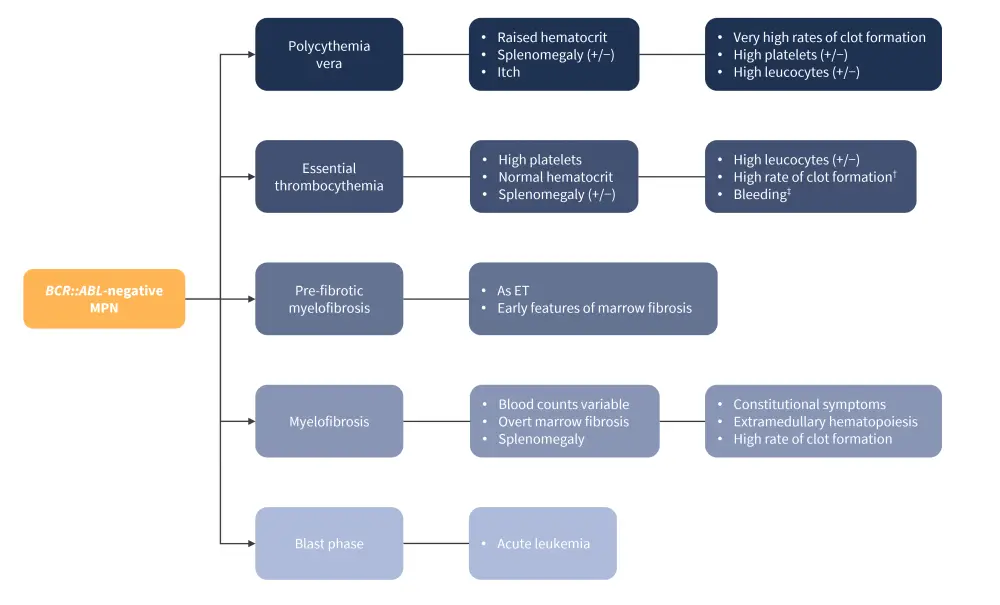
ET, essential thrombocytopenia; MPN, myeloproliferative neoplasm.
*Adapted from Greenfield, et al.1
Here, we provide a summary of MPN, including its etiology, epidemiology, pathophysiology, diagnosis, and treatment options.
Etiology
The genetic abnormalities commonly identified in patients with MPN are mutations in Janus kinase 2 (JAK2), calreticulin, and myeloproliferative leukemia virus oncogene.1
- Most patients present with the JAK2V617F mutation2:
- 95% of patients are diagnosed with PV
- 50% of patients are diagnosed with MF
- 50% of patients are diagnosed with ET
The JAK2 gene is essential for the normal cytokine signaling associated with the erythropoietin, thromboembolic, and granulocyte colony-stimulating factor receptors.1 Mutations remove the inhibitory effects of the JAK2 gene, resulting in:
- continuous activation of the STAT1 (signal transducers and activators of transcription 1), 3 and 5 transcription factors, phosphoinositide 3-kinase/protein kinase B, and mitogen-activated protein kinase signaling pathways; and
- proliferation, differentiation, and survival of myeloid cells at an increased rate.1
Patients with clonal hematopoiesis of indeterminate potential are at an increased risk of developing myeloid malignancies, including MPN.1 The risk of developing MPN has been increasingly associated with familial inheritance and germline disposition.1 Compared with other myeloid malignancies, the heritable risk of MPN is significantly higher.
Other non-genetic risk factors include a history of smoking, sex (higher prevalence of PV in males and higher prevalence of ET in females),2 and a higher JAK2V617F allele burden in males compared with females.1
Epidemiology
MPN is typically diagnosed in patients in their early 60s.3 Diagnoses in young adults (aged <40 years) are uncommon (Figure 2).2
Figure 2. Epidemiology of MPN*

*Data from Greenfield, et al.1; McMullin and Anderson.2; Li, et al.4; Hultcrantz, et al.12; Leukamia and Lymphoma Society.14.
†Equivalent to a healthy individual.
Pathophysiology1
The pathology of MPN is complex and multifactorial. All MPN originate at the level of pluripotent hematopoietic stem cells, with the key pathologic event being the constitutive activation of the Janus kinase/signal transducers and activators of transcription signaling pathway. Numerous cellular and metabolic functions, such as metabolism, cell cycle control, apoptosis, DNA damage responses, and direct or indirect transcription factor control, are linked to this pathway (Figure 3). There is compelling evidence to suggest overactive inflammation is another key contributor to MPN development.
Figure 3. Molecular pathogenesis of MPN*
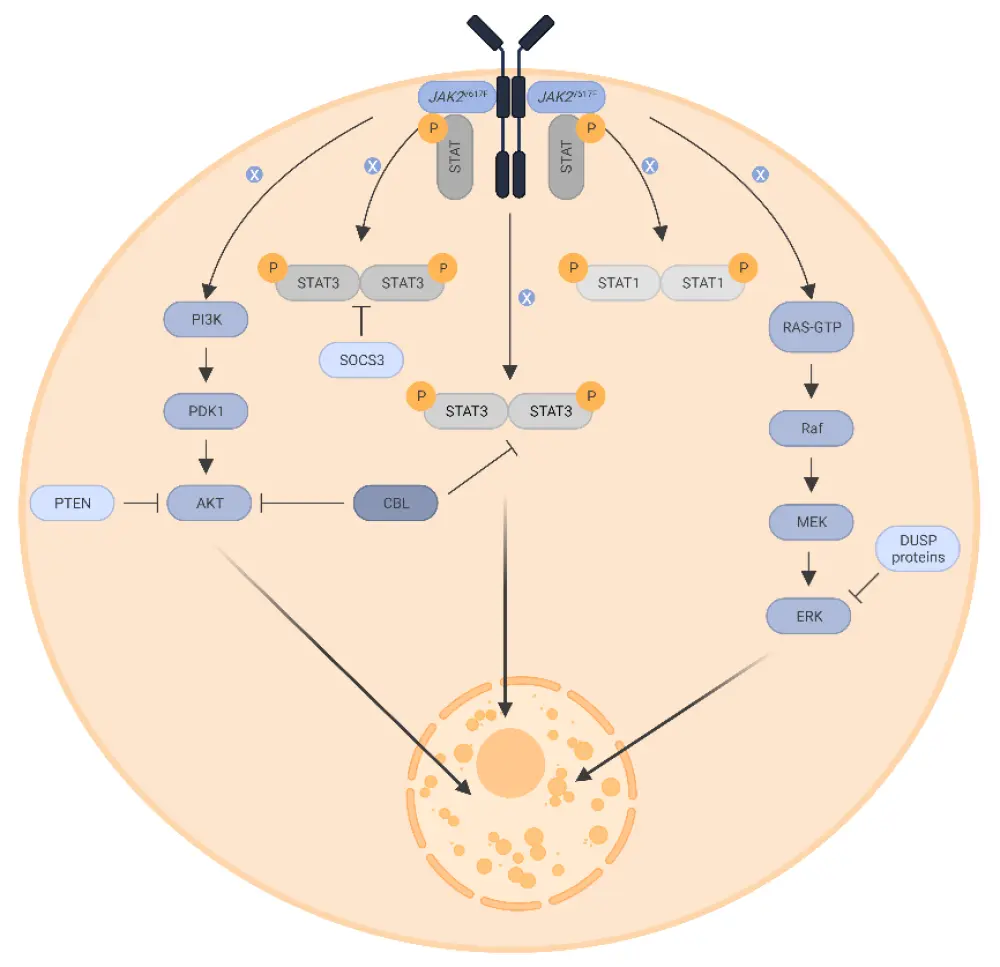
AKT, protein kinase B; CBL, casitas B cell lymphoma; ERK, extracellular signal-regulated kinase; DUSP, dual-specificity phosphatase; JAK, Janus kinase 2; MEK, mitogen-activated protein kinase kinase; PDK1, phosphoinositide-dependent kinase-1; PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homologue; Raf, rapidly accelerated fibrosarcoma; RAS-GTP, rat sarcoma virus-guanosine triphosphate; STAT, signal transducer and activator of transcription; SOCS3, suppressor of cytokine signaling 3.
*Adapted from Greenfield, et al.1 Created with BioRender.com.
Signs, symptoms, and risks
The general signs of a potential MPN diagnosis are shown in Figure 4.
Figure 4. General signs of MPN*
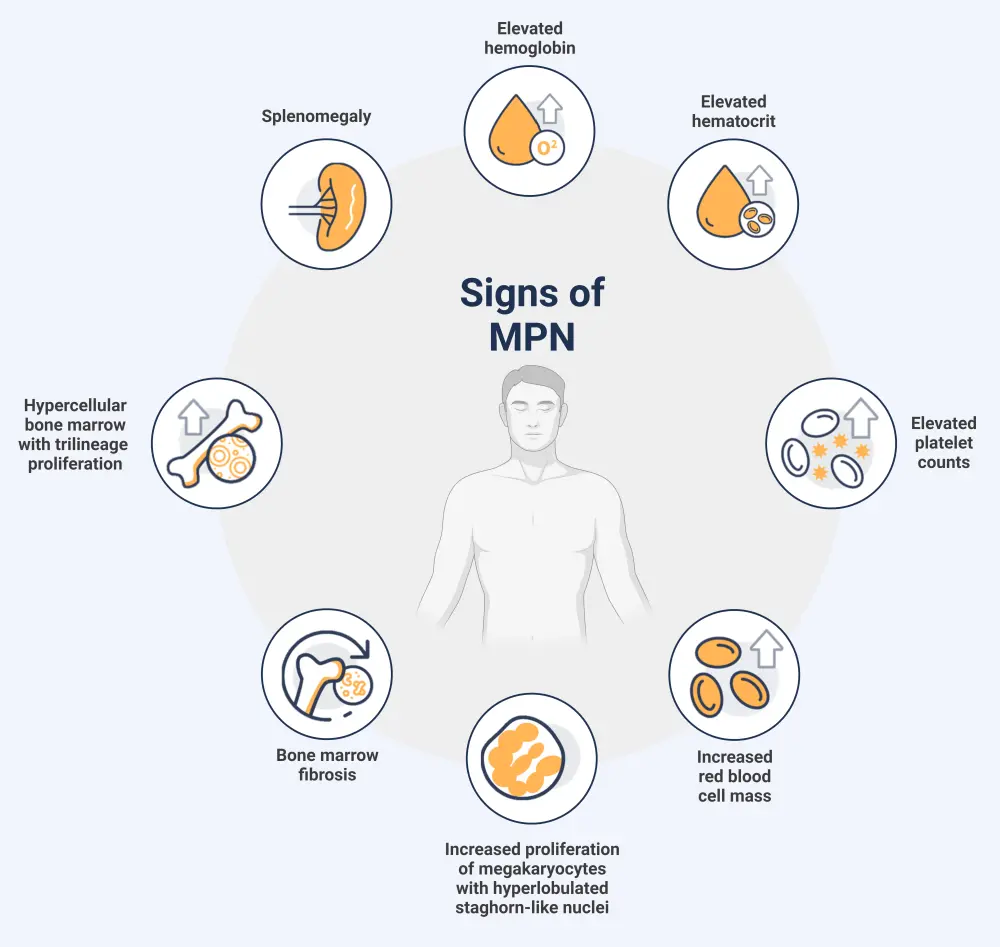
MPN, myeloproliferative neoplasm.
*Adapted from Tremblay.3 Created with BioRender.com.
The general constitutional symptoms of MPN are outlined in Figure 5.
Figure 5. General symptoms of MPN*
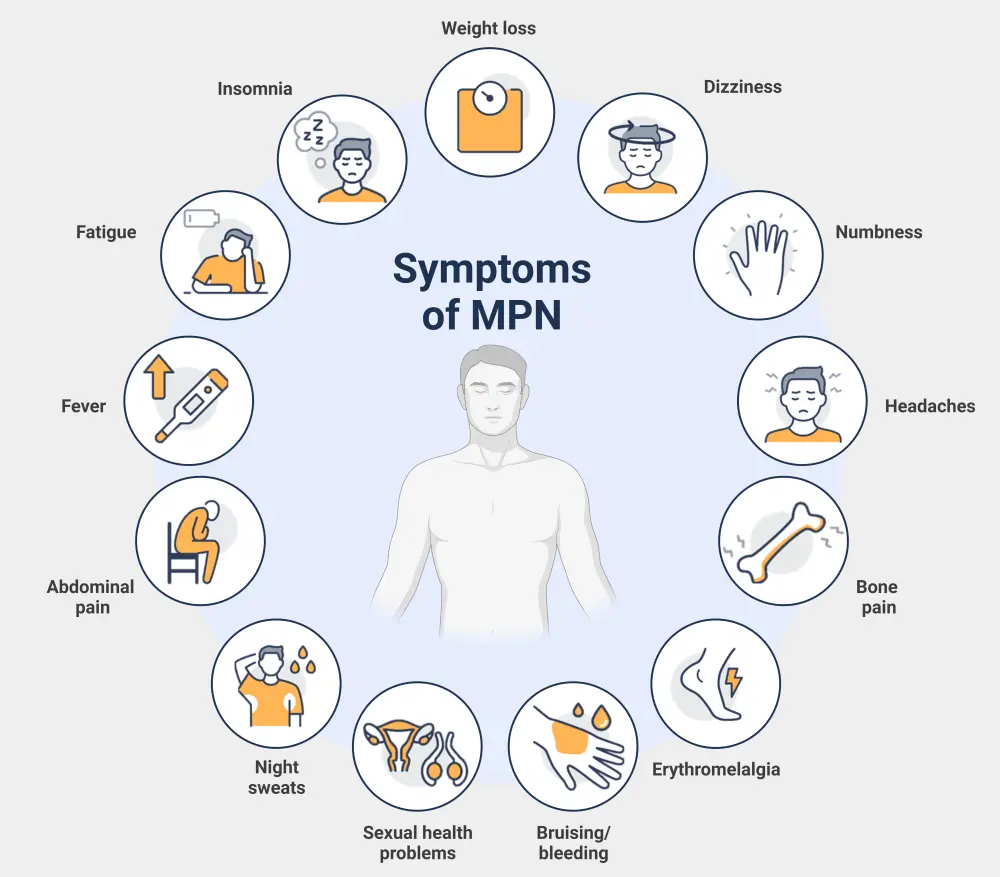
MPN, myeloproliferative neoplasms.
*Adapted from Rumi and Cazzola.6 Created with BioRender.com.
Patients with MPN are at a higher risk of thrombohemorrhagic events and progression to acute myeloid leukemia. Over time, the risk of both arterial and venous events increases.4
Diagnosis
The diagnosis of each MPN subtype has been historically defined by the World Health Organization (WHO) classification of MPN. However, the most recent revision has resulted in a new scheme called the International Consensus Classification of MPN, with an emphasis on criteria refinement for easier distinction between subtypes.
For a confirmed subtype diagnosis, a patient must meet all major criteria defined in the classification, or most of the major criteria together with a minor criterion (Figure 6).
Assessment of clinical and laboratory features through bone marrow biopsies, blood tests, and cytogenetic profiling is often necessary to ascertain a specific diagnosis. Guidance on diagnosis may vary between countries (see key guidelines section).
Figure 6. ICC of MPN major and minor diagnostic criteria*
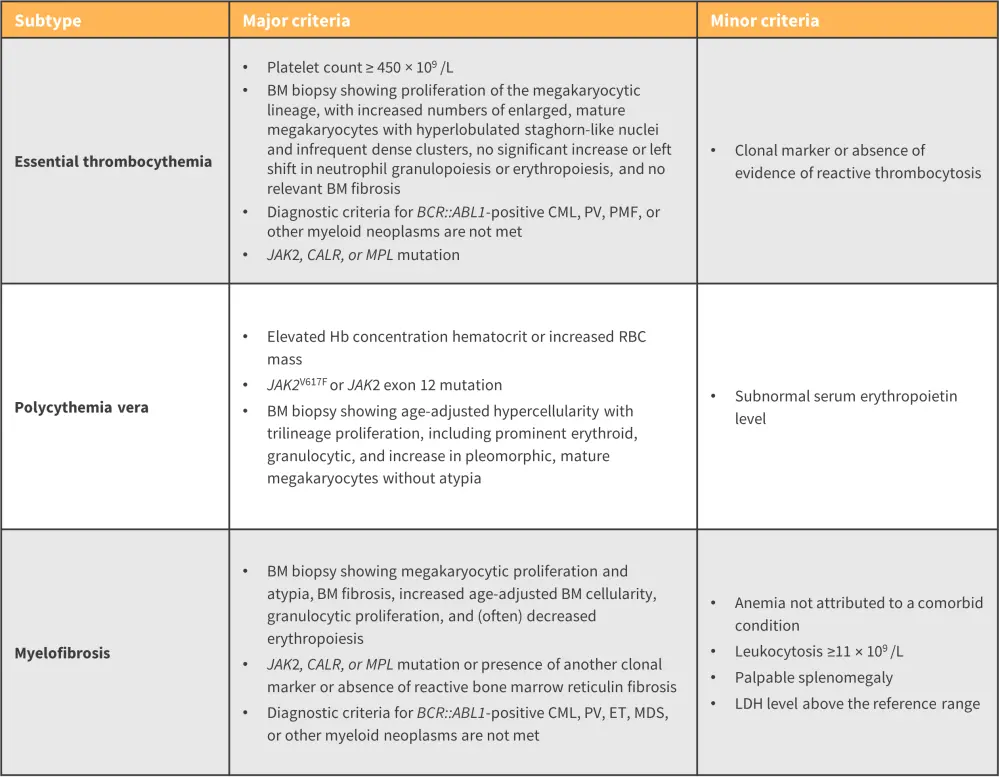
BM, bone marrow; CALR, calreticulin; CML, chronic myeloid leukemia; ET, essential thrombocythemia; Hb, hemoglobin; ICC, International Consensus Classification; JAK, Janus kinase; LDH, lactate dehydrogenase; MDS, myelodysplastic syndrome; MF, myelofibrosis; PMF, primary myelofibrosis; MPN, myeloproliferative neoplasms; PV, polycythemia vera; RBC, red blood cell; WBC, white blood cell.
*Adapted from Arber, et al.12
Risk stratification6
Several risk-stratification models are available for each MPN disease subtype to assess patient prognosis and guide treatment strategies.
- Models available for ET risk stratification include:
- The conventional score model (European LeukemiaNet)
- IPSET-Thrombosis (International Prognostic Score for Thrombosis)
- IPSET-Survival (International Prognostic Score for Survival)
- Models available for PV risk stratification include:
- The conventional score model (European LeukemiaNet)
- IPSS-OS (International Prognostic Scoring System for Overall Survival)
- Models available for MF risk stratification include:
- IPSS (International Prognostic Scoring System)
- DIPSS (Dynamic International Prognostic Scoring System)
- DIPSS+ (Dynamic International Prognostic Scoring System plus)
Management
Over the past 2 decades, the treatment options for patients diagnosed with MPN have rapidly expanded (Figure 7). For more information on the timeline of drug approvals over the last 20 years, check out our visual summary here.
The main treatment options for patients diagnosed with MF are Janus kinase inhibitors (JAKis). These therapies selectively inhibit one or more JAK proteins.7 The first approved JAKi was ruxolitinib, followed by fedratinib, pacritinib, and finally momelotinib, which was approved in September 2023.7JAKi treatment has a high risk of worsening anemia, which is already a challenge for patients with MF.8
- For patients who have developed resistance to JAKis and are diagnosed with persistent or progressive MF, navitoclax, a B-cell lymphoma 2 inhibitor can be added as a combination treatment option.7
- Pelabresib, a bromodomain and extra-terminal domain inhibitor, as both a single agent and in combination with ruxolitinib, is another option available for both first-line and refractory settings in MF.7
The first-line therapy for patients diagnosed with low-risk PV or ET is aspirin.5 The second-line treatment recommendation is cytoreductive therapies, including hydroxyurea (HU), or anagrelide.5
- Pegylated forms of interferon alfa-2a and interferon alfa-2b are recommended for patients who develop an intolerance of resistance to HU.5
- Intermediate- and high-risk patients are recommended HU as first-line treatment.5
Guidance on MPN management may vary between countries (see guidelines section).
Figure 7. Overview of treatment options available for patients diagnosed with MPN*
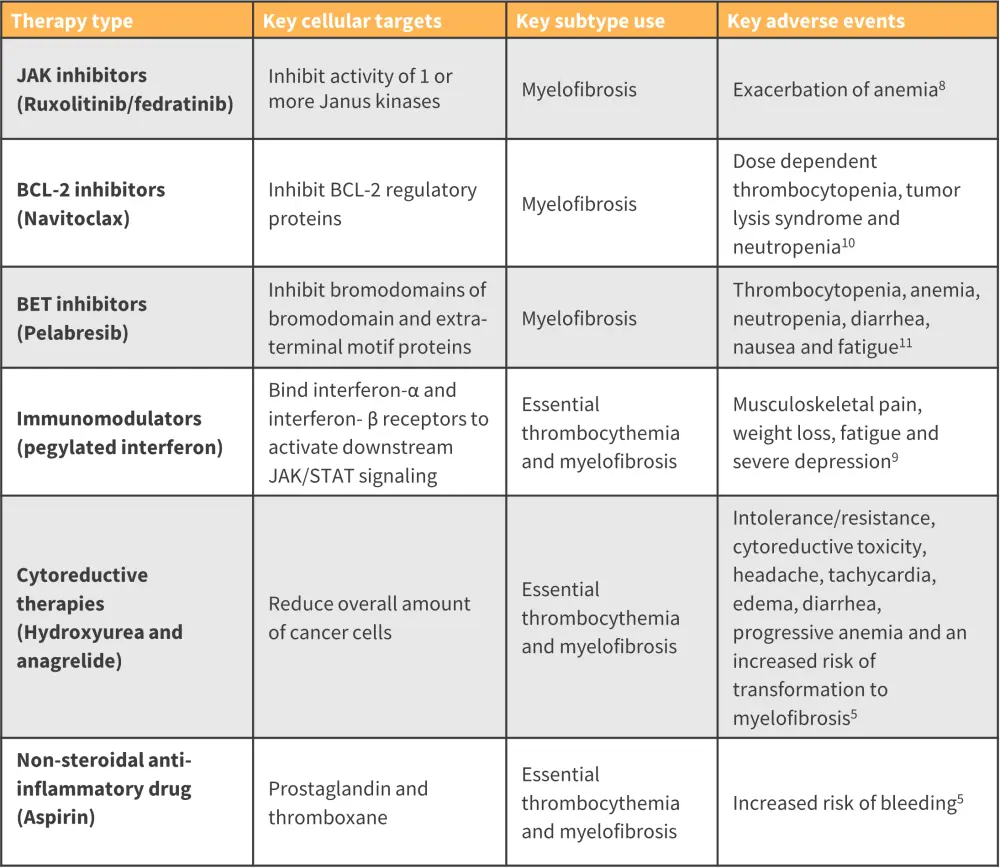
BET, bromodomain and extra-terminal motif; BCL-2, B-cell lymphoma 2; JAK, Janus kinase; JAK/STAT, JAK/ signal transducers and activators of transcription.
*Adapted from Khodier and Gadó.5; Passamonti, et al.8; Garmezy, et al.9; Lampson and Davids.10; Gangat and Tefferi.11
Key Guidelines and organizations
- European LeukemiaNet
- Leukemia and Lymphoma Society
- World Health Organization
- MPN Research Foundation
- MPN Hub key updates to the 5th WHO Classification of MPNs
- MPN Hub International Consensus Classification 2022
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

