All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
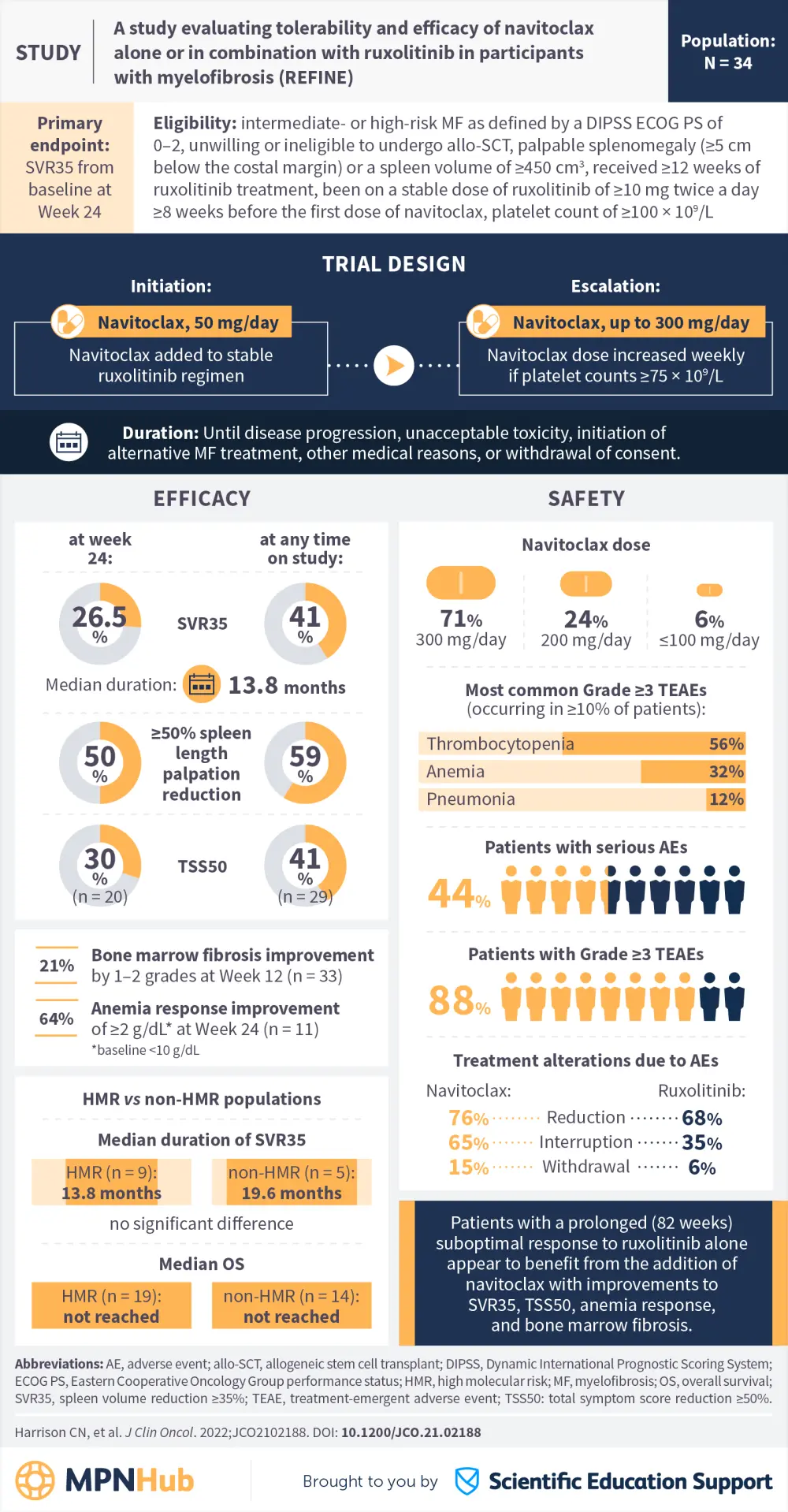
Navitoclax add-on to ruxolitinib in patients with myelofibrosis: Latest data from the phase II REFINE trial
In March 2022, Mohamad Mohty, Hôpital Saint-Antoine and Sorbonne University, Paris, FR, held a live virtual Journal Club, organized by the International Academy for Clinical Hematology (IACH), joined by Naveen Pemmaraju, MD Anderson Cancer Center, Houston, US, and Ibrahim Yakoub-Agha, CHU de Lille, Lille, FR.1 The panel discussed the results of the recently published phase II REFINE trial (NCT03222609)2 on the efficacy and safety of the addition of navitoclax to ruxolitinib in patients with persistent or progressive myelofibrosis. The MPN Hub is pleased to summarize highlights from the event, below.
- Ibrahim Yakoub-Agha shared his observation on (i) the study population not being fully representative of hard-to-treat patients based on the International Prognostic Scoring System (IPSS) or the Dynamic International Prognostic Scoring System (DIPSS) definition (44% of patients had intermediate-1-risk disease), and (ii) the number of patients proceeding to transplant being very low (2/34)
-
- For the first point of discussion, Naveen Pemmaraju explained that over half of patients had single or multiple high-molecular risk (HMR) mutations, thus these patients could be considered as a high-risk population in different terms (i.e., based on molecular mutations). Concerning bridge to transplant, he pointed out that not all patients were eligible for transplant; the study population included patients with progressive disease, older patients, and deaths and withdrawals occurred. Out of the remaining patients, some may be more likely to proceed to transplant in the longer term (this topic will be in the scope of the hub’s future updates)
- Ibrahim Yakoub-Agha also highlighted the challenges on allogeneic transplant: over half of the patients included in the study were >68 years, and there were comorbidities, issues with eligibility, fitness for transplant, and donor availability.
- The panel discussed the safety profile of the combination treatment with two main adverse events (AEs) of interest: thrombocytopenia and gastrointestinal signals (including diarrhea, vomiting, and nausea). Naveen Pemmaraju clarified that the rate of thrombocytopenia was high, but these events were reversible with no bleeding or major hemorrhages, and they also monitored the overlapping toxicity with ruxolitinib with dose modifications (escalation and de-escalations) based on platelet counts. He highlighted that a weekly check of the platelet count may be needed for the first several weeks of treatment. He also mentioned that TRANSFORM-1 (NCT04472598) and TRANSFORM-2 (NCT04468984) randomized phase III trials have been recently launched to evaluate the combination navitoclax plus ruxolitinib in frontline settings, placebo-based combinations, and second-line settings which would provide additional insights into the safety profile of the combination therapy.
Overall, the panel emphasized that despite the relatively low number of patients, the preliminary results published in the study are encouraging. Combination therapy of navitoclax with ruxolitinib may provide a novel treatment approach for patients with suboptimal response to Janus kinase (JAK) inhibitors. The study can pave the way for further investigation on combination therapies for patients with persistent or progressive myelofibrosis.
Visual abstract
Below, we provide a visual abstract summarizing key efficacy and safety results from the REFINE trial, which were recently published in the Journal of Clinical Oncology.2
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

