All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Review | Biology, diagnosis, and treatment of myelofibrosis up to date
Recently, the British Journal of Haematology published a review on myelofibrosis by Naseema Gangat and Ayalew Tefferi from Mayo Clinic, Rochester, US.1 This article summarizes the key aspects of biological pathogenesis, their recommendations on the current management of the disease, and new therapies in clinical development.
Classification1
According to the World Health Organization (WHO) in 2016, the disease broadly termed as myelofibrosis, and identified as one of the Philadelphia chromosome-negative myeloproliferative neoplasms (MPN), can be further classified as
- prefibrotic myelofibrosis
- primary myelofibrosis (PMF) or overly fibrotic myelofibrosis
- secondary myelofibrosis or cases transformed from polycythemia vera (PV) and essential thrombocytopenia (ET; post-PV/ET MF)
Disease biology and pathogenesis1
Like other Philadelphia-negative MPN, the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway is constitutively activated in aberrant megakaryocytes by different mechanisms. The most frequently described mutations, reported alone or concomitant, in myelofibrosis are
- JAK2 V617F mutation: ~ 60%
- calreticulin (CALR) mutations: 23%
- thrombopoietin (TPO) receptor, myeloproliferative leukemia virus (MPL) mutations: 7%
- triple-negative status: 10%
- All mutations either hinder the inhibition of JAK/STAT signaling or directly increase its activity. The hyperactivation of this proliferative pathway ultimately results in the uncontrolled activation of aberrant megakaryocytes, with release of pro-inflammatory cytokines and the subsequent increase of bone marrow fibrosis. In later stages of the disease, the bone marrow fibrosis progresses to collagen fibrosis and osteosclerosis. Find more specific information on genetic, epigenetic, and cell aberrations in Table 1.
Table 1. Mechanisms of disruption of JAK/STAT signaling and its consequences in aberrant megakaryocytes described in patients with myelofibrosis1
|
CALR, calreticulin; HSC, hematopoietic stem cell; IDH, isocitrate dehydrogenase; IL, interleukin; IP, induced protein; JAK, Janus kinase; MMP, matrix metalloproteinase; MPL, myeloproliferative leukemia virus; mRNA, messenger RNA; PMF, primary myelofibrosis; PV, polycythemia vera; STAT, signal transducer and activator of transcription; TGF, transforming growth factor; TPO, thrombopoietin |
|
|
Described driver mutations |
|
|---|---|
|
JAK2 V617F mutation |
|
|
MPL mutations |
|
|
CALR mutations |
|
|
Triple-negative |
|
|
Other cell aberrations |
|
|
Mutated epigenetic regulators |
|
|
Splicing factor mutations |
|
|
GATA1 low expression |
|
|
IDH mutations |
|
|
Megakaryocytic hyperplasia and resulting bone marrow fibrosis |
|
|
Bone marrow microenvironment |
|
Diagnosis and prognostic assessment1
Clinical characteristics and laboratory results at diagnosis
Clinical symptoms frequently observed at myelofibrosis diagnosis:
- Palpable splenomegaly in 72% of patients
- Constitutional symptoms in 29% of cases: fatigue, night sweats, weight loss, or cachexia
- Symptomatic or transfusion-dependent anemia in 33% of patients
- Pruritus
- Thrombosis
- Hemorrhage
- Recurrent infections
Laboratory findings:
- Leucoerythroblastic smear with dacrocytosis
- Nucleated red cells
- Leukocytosis ±
- circulating blasts and thrombocytosis, or
- thrombocytopenia with circulating megakaryocytes
- Bone marrow sample could be challenging to obtain depending on the fibrotic stage
- Presence of a driver mutation (JAK2, CALR, or MPL)
Disease prognosis
Several tools for myelofibrosis prognostic assessment have been developed, and the original publication reviews the evolution of the International Prognostic Scoring System (IPSS).1 This article will focus only on the current IPSSs recommended by the authors to identify patients with PMF for allogeneic transplant: the mutation and karyotype-enhanced IPSS for age ≤ 70 years (MIPSS70-plus version 2.0) and the genetically-inspired scoring system for all age groups (GIPSS)(Table 2).
Table 2. MIPSS70-plus version 2.0 and GIPSS1
|
CALR, calreticulin; GIPSS, genetically-inspired scoring system for all age groups; HMR, high molecular risk; MIPSS 70-Plus, mutation and karyotype-enhanced international prognostic scoring system for age ≤ 70 years; NR, not reached; OS, overall survival; p, points * Very high risk karyotype includes single/multiple abnormalities of -7, inv(3)/3q21, i(17q), 12p-/12p11.2, or 11q-/11q23, single/multiple autosomal trisomies other than +9 and +8. † Unfavorable karyotype comprises of any abnormal karyotype other than normal karyotype or sole abnormalities of 20q-, 13q-, +9, chromosome 1 translocation/duplication, -Y, or sex chromosome abnormality other than -Y. ‡ HMR mutations include ASXL1, EZH1, SRSF2, IDH1, IDH2, and U2AF1Q157. |
|
|
MIPSS70-plus version 2.0 (online calculator) |
|
|---|---|
|
Variables |
Identified groups and survival outcomes |
|
Severe (2 p) or moderate (1 p) anemia |
0 p = very low risk; median OS, NR |
|
Circulating blasts ≥ 2% (1 p) |
1–2 p = low risk; median OS, 16.4 years |
|
Constitutional symptoms (2 p) |
3–4 p = intermediate risk; median OS, 7.7 years |
|
Very high risk* (4 p) or unfavorable† (3 p) cytogenetics |
5–8 p = high risk; median OS, 4.1 years |
|
≥ 2 HMR‡ mutations (3 p) One HMR mutation (2 p) Type 1-like CALR absent (2 p) |
≥ 9 p = very high risk; median OS, 1.8 years |
|
GIPSS |
|
|
Variables |
Identified groups and survival outcomes |
|
Very high risk (2 p) or unfavorable (1 p) cytogenetics |
0 p = very low risk; median OS, 26.4 years |
|
ASXL1 (1 p) |
1 p = low risk; median OS, 8 years |
|
SRSF2 (1 p) |
2 p = high risk; median OS, 4.2 years |
|
U2AF1Q157 (1 p) |
≥ 3 p = very high risk; median OS, 2 years |
|
Type 1-like CALR absent at any time (1 p) |
|
Following transplantation, other prognostic tools can be used to predict disease evolution, such as the recently developed myelofibrosis transplant scoring system (MTSS), which includes clinical and laboratory features to identify four different risk groups and their survival probability at 5 years since diagnosis. This model was developed with data from 361 patients with PMF and secondary myelofibrosis and validated in a cohort of 156 patients (Figure 1).2
Figure 1. Myelofibrosis transplant scoring system (MTSS) to predict outcome after allogeneic transplantation2
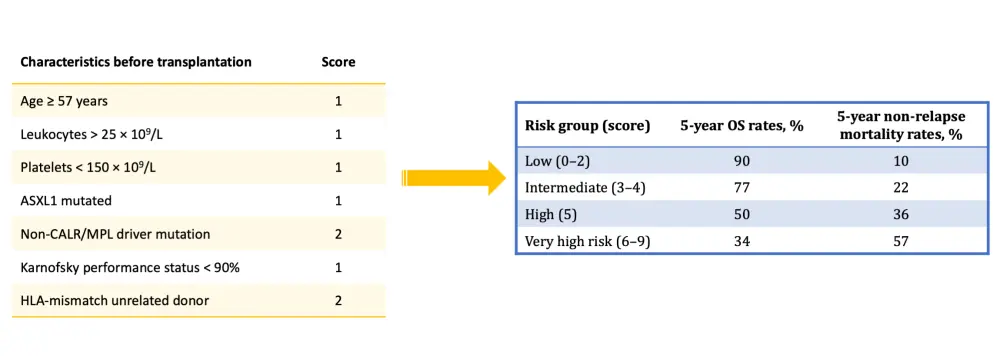
Additionally, there has been significant progress in formulating a model to predict myelofibrosis conversion from chronic to blast phase. This transformation is infrequent (10–20% of patients), but it has a significant impact on survival. The Mayo Clinic recently published a predictive model for blast transformation and found that high-risk karyotype, platelet count, transfusion need, and age were independent predictors of survival after blast transformation. This model identifies high-, intermediate-, and low-risk of blast transformation subgroups with an incidence of 57%, 17%, and 8%, respectively.3
Clinical management
To date, treatment strategies do not distinguish between primary or secondary myelofibrosis and are mostly disease modifying, with the exception of allogeneic stem cell transplantation (allo-SCT), which is the only curative option. So far, however, allo-SCT has been reserved for patients < 70 years old, but this might change with the extended use of MIPSS70-plus helping to identify older patients who are eligible for transplant.
Transplantation, new therapies, and supportive care altogether improve the outcomes of patients with myelofibrosis, but the disease evolution is still very heterogeneous, and thus, median survival ranges from > 10 years to < 2 years.
Several drugs are currently available or in clinical development to palliate determinant symptoms or to slow disease progression. Patients that are asymptomatic and classified as very low or low risk are usually maintained under observation. Therapy is chosen for symptomatic patients according to the predominant symptom at the time of therapy initiation (Figure 2), which predominantly include
- Symptomatic anemia: Usually managed with recurrent transfusions combined with erythropoietin stimulating agents, androgens (danazol), and immunomodulators (thalidomide or lenalidomide) with prednisone; however, myelofibrosis-related anemia is still an unmet need
- Splenomegaly: Frequently palliated with hydroxyurea, and if intolerant or refractory, with the approved JAK2 inhibitors (ruxolitinib or fedratinib). When splenomegaly is refractory to drugs, the authors recommend splenectomy or splenic radiation
- Constitutional symptoms: Effectively and rapidly controlled with ruxolitinib in most patients, although it is essential to consider the higher incidence of significant anemia (33%), thrombocytopenia (26%), opportunistic infections, and withdrawal symptoms (11%) under ruxolitinib therapy
Figure 2. Treatment approach of myelofibrosis according to prognostic risk group1
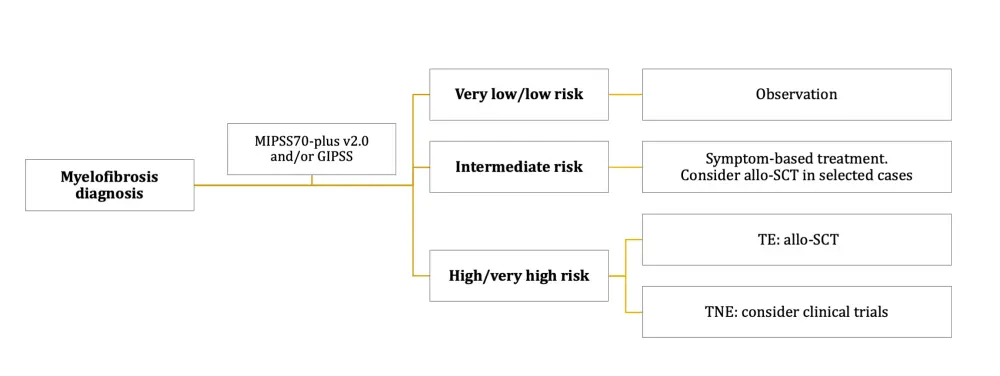
The approval of JAK2 inhibitors for patients with myelofibrosis had a considerable impact on the management of disease (see the approval of ruxolitinib in 2011 and, more recently, fedratinib). However, patients still relapse after a median of 2–3 years on treatment, and outcome after relapse to ruxolitinib is poor. Table 3 summarizes the latest results of the most promising novel JAK2 inhibitors and other targeted therapies being investigated as alternatives, in combination, or as a subsequent treatment to ruxolitinib for patients with myelofibrosis. Find a recent review of these and other selected novel agents for myelofibrosis in clinical development, here.
Table 3. Novel targeted drugs in clinical development for patients with primary and secondary myelofibrosis1
|
ACVR1, activin A type 1 receptor; AE, adverse event; ASH, American Society of Hematology; BCL-2, B-cell lymphoma 2; BET, bromodomain and extraterminal domain protein; CLS; capillary leak syndrome; EHA, European Hematology Association; FLT3, fms-like tyrosine kinase 3; IL-3, human interleukin-3; IRAK1, interleukin-1 receptor-associated kinase 1; JAK, Janus kinase; LSD, Lysine-specific demethylase; MF, myelofibrosis; PMF, primary myelofibrosis; TGF, transforming growth factor; TRAE, treatment-related adverse event |
|
|
Novel JAK2 inhibitors |
|
|---|---|
|
Momelotinib
|
|
|
Pacritinib
|
|
|
Other novel therapies for myelofibrosis |
|
|
Metformin
|
|
|
Azacitidine
|
|
|
Bomedemstat (IMG-7289)
|
|
|
Luspatercept
|
|
|
Tagraxofusp
|
|
|
CPI-0610
|
|
|
Navitoclax
|
|
Conclusion
There have been remarkable advances made in the understanding of molecular and pathological processes involved in the development and progression of myelofibrosis. Ongoing research will help to elucidate those mechanisms further and identify future targets for the treatment of primary and secondary myelofibrosis and their disease-related complications.
With the prognostic models used at present, it is possible to stratify patients with myelofibrosis according to their risk of progression and the urgency of treatment initiation. Currently, physicians can offer a curative goal to transplant-eligible patients only, and additional studies are needed to explore early intervention with novel therapies and allo-SCT, especially in high-risk patients.
As the median age of diagnosis of myelofibrosis is 65 years, access to transplantation is limited for most newly-diagnosed patients. Transplant-ineligible patients are treated with symptom control and palliative measures. Due to the limited efficacy of current treatment options, clinical trial enrollment is recommended, particularly for patients with intermediate/high-risk myelofibrosis.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

