All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Novel treatments: Momelotinib and luspatercept
Do you know... Momelotinib and luspatercept have a potentially synergistic mechanism of action in patients with myelofibrosis and anemia. What is their potential combined effect in these patients?
During the MPN Hub Steering Committee meeting, Ruben Mesa discussed the use of momelotinib and luspatercept for the treatment of patients with myelofibrosis and anemia, featuring John Mascarenhas, Steffen Koschmieder, Laura Michaelis, and Tiziano Barbui.
Novel treatments: Momelotinib and luspatercept
Novel treatments: Momelotinib and luspatercept
Mesa first discusses current and emerging therapies for patients with myelofibrosis (MF)-related anemia, including momelotinib and luspatercept, and highlights that both of these treatments are included in the British Society of Haematology (BSH) and the National Comprehensive Cancer Network (NCCN) guidelines for the treatment of patients with MF and anemia.1,2
Momelotinib
Momelotinib inhibits both the BMP/ACVR1/SMAD pathway and the IL-6/JAK/STAT3 pathway, resulting in a decrease in hepcidin, which may improve anemia in patients with MF.3
Three key phase III trials have assessed the efficacy and safety of momelotinib in patients with MF: SIMPLIFY-14 (NCT01969838), SIMPLIFY-25 (NCT02101268), and MOMENTUM6 (NCT04173494).
In the SIMPLIFY-1 trial, momelotinib demonstrated noninferiority vs ruxolitinib in terms of 35% reduction in spleen volume (SVR35) response (26.5% vs 29.0%; p = 0.011), while transfusion independence (TI) rates were improved (Figure 1).4
Anemia was observed more frequently in the ruxolitinib arm (38.0%) vs the momelotinib arm (13.6%).4
Figure 1. TI rates in the SIMPLIFY-1 trial*
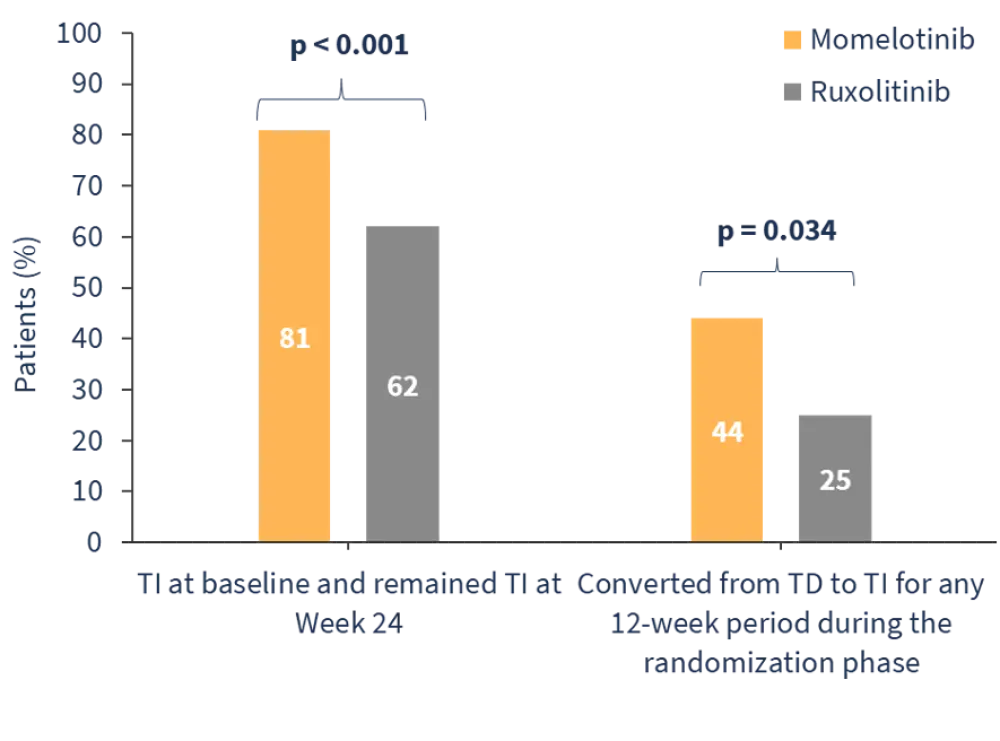
In the SIMPLIFY-2 trial, although SVR35 response rates were similar, momelotinib improved TI rates vs best available therapy (43% vs 21%; p = 0.012).5
A pooled survival analysis from the SIMPLIFY trials suggested that momelotinib was associated with a survival benefit in patients who became TI.8
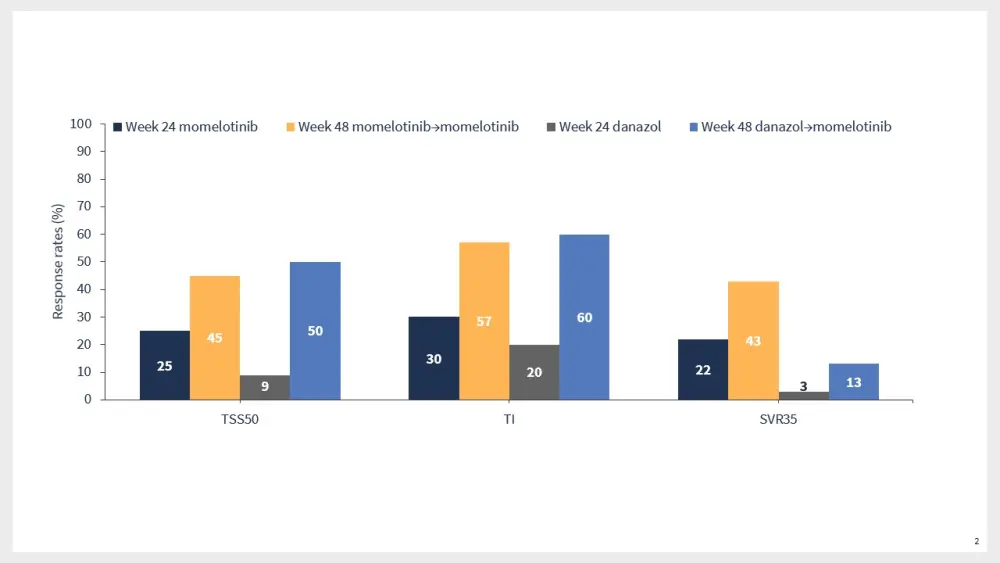
Results from the MOMENTUM trial suggested that momelotinib was associated with durable symptom, TI, and SVR35 responses vs danazol (Figure 2).9
Figure 2. Key efficacy outcomes in patients who continued momelotinib treatment or crossed over from danazol in the MOMENTUM trial*
Luspatercept
Luspatercept is an activin receptor ligand trap that is approved for the treatment of patients with myelodysplastic syndromes.10
Luspatercept binds tumor growth factor β superfamily ligands to diminish SMAD2/3 signaling and enhance late-stage erythropoiesis.11
The phase II ACE-536-MF-001 trial (NCT03194542) assessed the efficacy and safety of luspatercept in patients with MF and anemia, with and without both transfusion dependence (TD) and concomitant ruxolitinib treatment.11
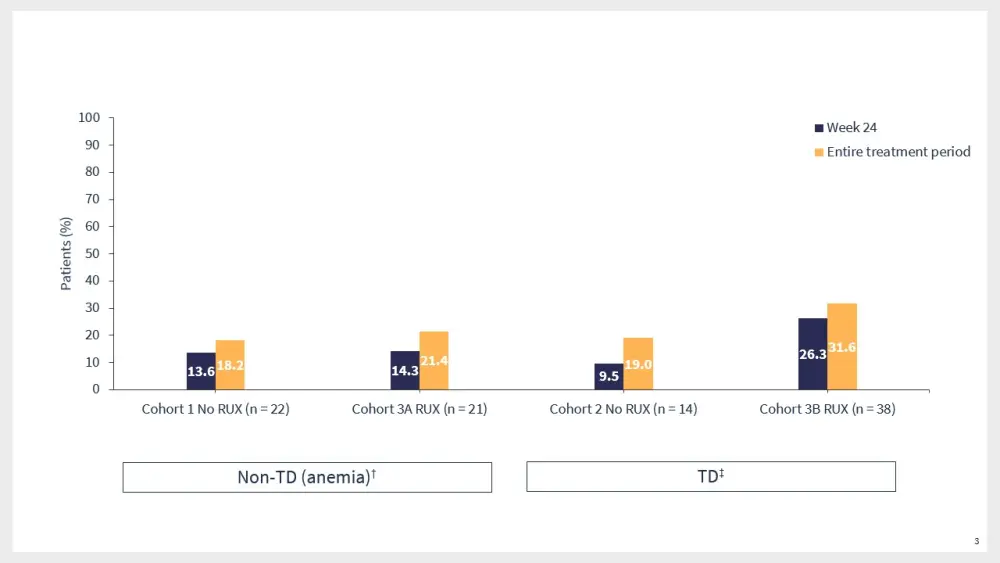
Results from the ACE-536-MF-001 trial demonstrated that luspatercept was associated with anemia response in both TD and non-TD patients (Figure 3).11
Luspatercept also improved mean hemoglobin rates in non-TD patients, and the transfusion burden in TD patients.11
The safety profile of luspatercept was consistent with previous studies.11
Figure 3. Anemia response rates in the ACE-536-MF-001 trial*
The ongoing phase III INDEPENDENCE trial (NCT04717414) is assessing the efficacy and safety of luspatercept in combination with ruxolitinib in patients with MF.12
Momelotinib and luspatercept have a complementary mechanism of action, which may improve anemia by promoting both early- and late-stage erythropoiesis, suggesting a potential additive and possibly synergistic benefit.13
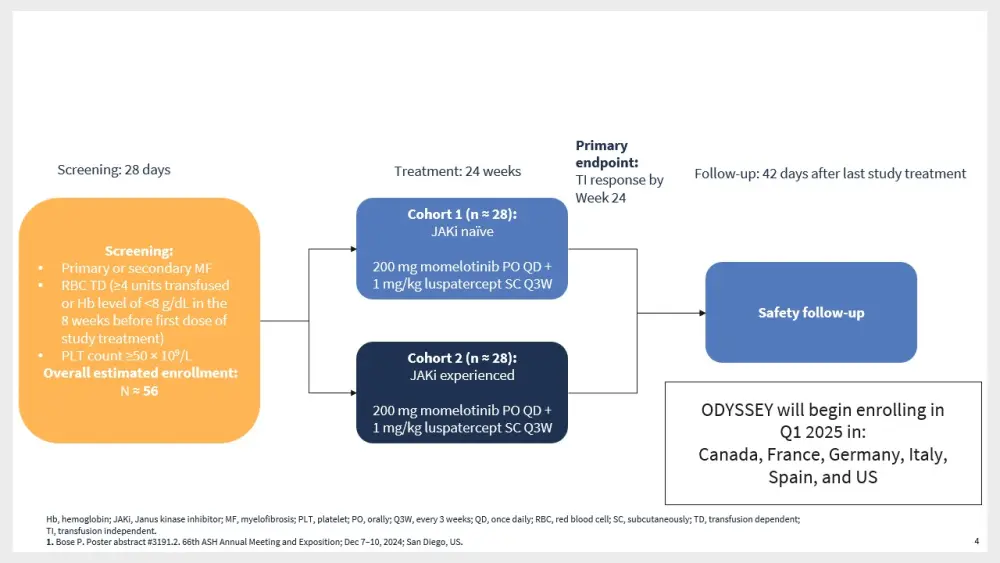
The phase II ODYSSEY trial (NCT06517875) will assess the combination of momelotinib and luspatercept in TD patients with MF (Figure 4).13
Figure 4. ODYSSEY study design*
Discussion
Key discussion points:
The panel discussed the pooled survival analysis from the SIMPLIFY trials, highlighting the impact of achieving TI on survival in patients treated with momelotinib, the potentially increased TD rates in patients who responded to ruxolitinib, the impact of dose levels, and iron overload.
Responses based on luspatercept dose were discussed, and it was suggested that patients tend to have a better response at a dose of 1.75 mg compared with lower doses.
The impact of concomitant leukopenia and thrombocytopenia was raised, and it was highlighted that momelotinib showed relative equivalence in terms of response in patients with platelet counts <50 × 109/L.
This educational resource is independently supported by GSK and Bristol Myers Squibb. All content was developed by SES in collaboration with an expert steering committee. Funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 John Mascarenhas
John Mascarenhas Ruben A. Mesa
Ruben A. Mesa Tiziano Barbui
Tiziano Barbui Laura Michaelis
Laura Michaelis Steffen Koschmieder
Steffen Koschmieder
