All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
One thousand patients with ET: Patient characteristics, management, and clinical outcomes
Essential thrombocythemia (ET) is a Philadelphia-chromosome-negative myeloproliferative neoplasia which is characterized by the abnormal proliferation of megakaryocytes in the bone marrow resulting in thrombocytosis.1
Gangat et al.1 and Loscocco et al.2 conducted retrospective studies documenting the patient characteristics, management strategies and clinical outcomes of 1,000 patients with ET at single centers in the United States (US) and Italy, respectively. The MPN Hub is pleased to summarize the results of these studies, with comparisons made between the two geographical centers.
Patient characteristics1,2
- In both the US and Italian cohorts:
- ET was more commonly observed in female patients than male (63% vs 37% and 65% vs 35% of patients, respectively); and
- cardiovascular risk factors were observed in over 50% of the patients.
- Extreme thrombocytosis, defined by a platelet count ≥1,000 × 109/L, was more commonly observed in the US cohort than in the Italian clinic with a prevalence of 26% vs 16%, respectively.
Additional patient characteristics, by region, are shown in Figure 1.
Figure 1. Patient characteristics by geographical region*
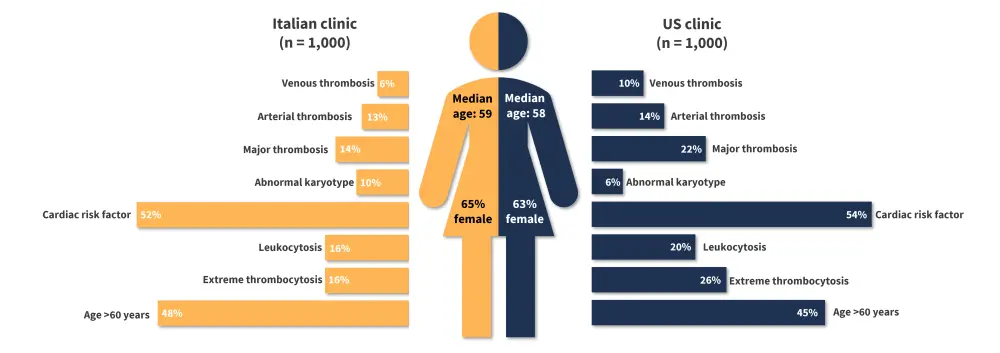
*Data from Gangat, et al.1 and Loscocco, et al.2
Driver mutations
Janus kinase 2 gene mutations are the most common driver mutation in myeloproliferative neoplasms, accounting for 62% and 66% of patients with ET in the US and Italian centers. Across both centers the distribution of driver mutations was comparable (Figure 2) with the order of most common mutations in both being:
- Janus kinase 2 mutation
- Calreticulin mutation
- Triple-negative
- Myeloproliferative leukemia
Figure 2. Distribution of driver mutations in 1,000 patients with ET from centers in A US and B Italy*
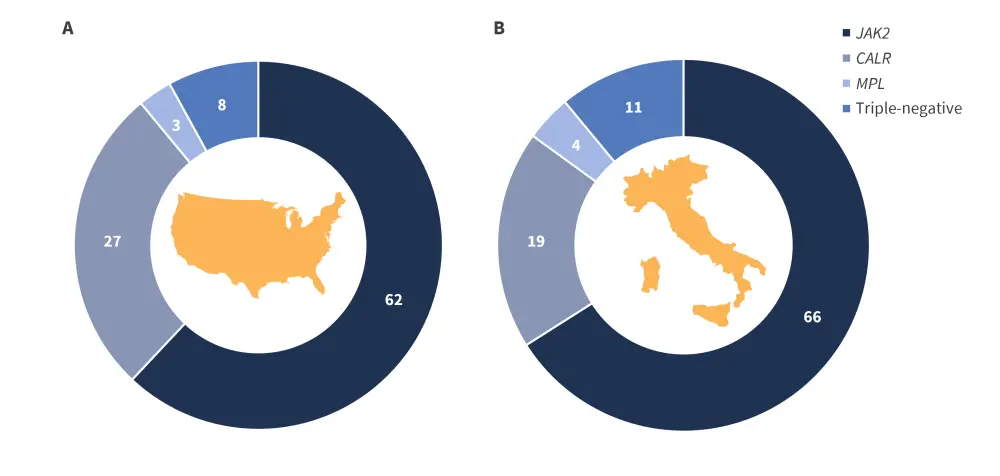
CALR, calreticulin; JAK, Janus kinase; MPL, myeloproliferative leukemia.
*Data from Gangat, et al.1 and Loscocco, et al.2
Treatment at diagnosis1,2
In both centers, initial treatment following diagnosis was comparable. The most common initial treatment in both cohorts was antiplatelet therapy (90%) or specifically aspirin (81%) in the US followed by cytoreductive therapy administered to >50% of patients (Figure 3).
Figure 3. Treatment instituted at diagnosis by region*
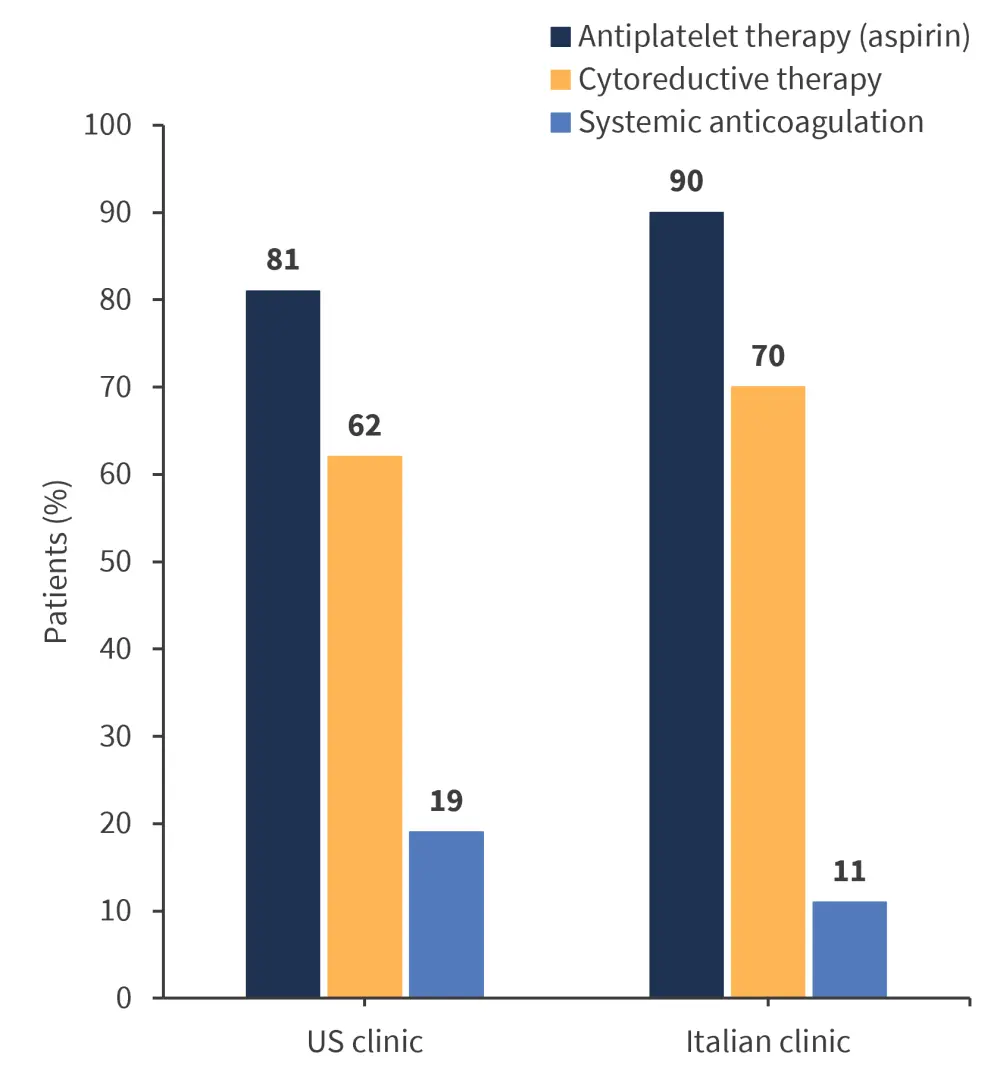
*Data from Gangat, et al.1 and Loscocco, et al.2
- Aspirin was identified to reduce the recurrence of arterial thrombosis
- Treatment with cytoreductive therapy was also found to be related to a reduced incidence of:
- venous thrombosis, hazard ratio 0.3 (p = 0.01); and
- arterial thrombosis, hazard ratio 4 (p = 0.04).
Outcomes1,2
Overall survival
- The risk factors identified for poorer overall survival were similar in both clinics with older age being most consistently associated with shorter survival periods.
- The impact of geographic location and risk factors on overall survival was also similar between cohorts (Table 1).
Table 1. Hazard ratios for overall survival by risk factor and geographic location*
|
*Data from Gangat, et al.1 and Loscocco, et al.2 |
||
|
Hazard ratio |
US clinic |
Italian clinic |
|---|---|---|
|
Male gender |
1.8 |
1.9 |
|
Absolute neutrophil count |
1.6 |
1.8 |
|
Absolute lymphocyte count |
1.5 |
1.2 |
|
Hypertension |
1.7 |
– |
|
Arterial thrombosis history |
1.7 |
– |
- The mortality rate during the follow-up period was 17% in the Italian center and 28% in the US.
- The most common causes of death in both groups included blast transformation, infection, thrombosis, solid tumors, major hemorrhage, heart failure, myelofibrosis, dementia, renal failure, hepatic failure, injury, and graft-versus-host disease.
Survival data over time, by region, is presented in Table 2.
Table 2. Overall survival in patients with ET by region*
|
ET, essential thrombocythemia; OS, overall survival. |
||
|
Survival, % (unless otherwise specified) |
US clinic |
Italian clinic |
|---|---|---|
|
20.6 |
27.1 |
|
|
10-year OS |
81 |
86 |
|
20-year OS |
52 |
64 |
|
30-year OS |
25 |
43 |
Conclusion
The trends in characteristics, management strategies and outcomes for patients with ET were generally consistent across the two geographical regions. Data from additional regions would enable further development of the reference bank, aiding in treatment development and ensuring representation of patient cohorts in pivotal clinical trials.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



