All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Polycythemia vera: Disease and treatment overview
Do you know... What is the most common genetic aberration identified in patients diagnosed with PV?
Polycythemia vera (PV) is a chronic Philadelphia chromosome-negative myeloproliferative neoplasm (MPN). It is indolent when compared with the other MPN disease subtypes, such as essential thrombocythemia and myelofibrosis.1 PV is characterized by uncontrolled clonal proliferation of myeloid blood cells, specifically of the erythropoietic lineage.2 In most patients, this is caused by a Janus kinase 2 (JAK2) mutation and results in erythrocytosis and bone marrow hypercellularity.3 Here, we provide an overview of the epidemiology, pathophysiology, diagnosis, and management of PV.
Etiology
- Approximately 90% of patients diagnosed with PV harbor a JAK2 mutation.2
- the most common mutation is JAK2V617F
- Around 2% of patients exhibit a mutation at exon 12 of JAK2.2
- In healthy individuals, the JAK2 gene is responsible for the activation of intracellular signaling involved in cell proliferation regulation, DNA damage repair, and cellular apoptosis.5
- The presence of a mutation leads to loss of the normal inhibitory function of JAK2, and results in constitutive activation of the JAK/signal transducers and activators of transcription and phosphoinositide 3-kinase/mitogen-activated protein kinase signaling pathways.5
- Risk factors for PV include:
- working in agriculture and petroleum refineries;
- benzene exposure; and
- smoking.6
Epidemiology
Figure 1. Polycythemia vera epidemiology*
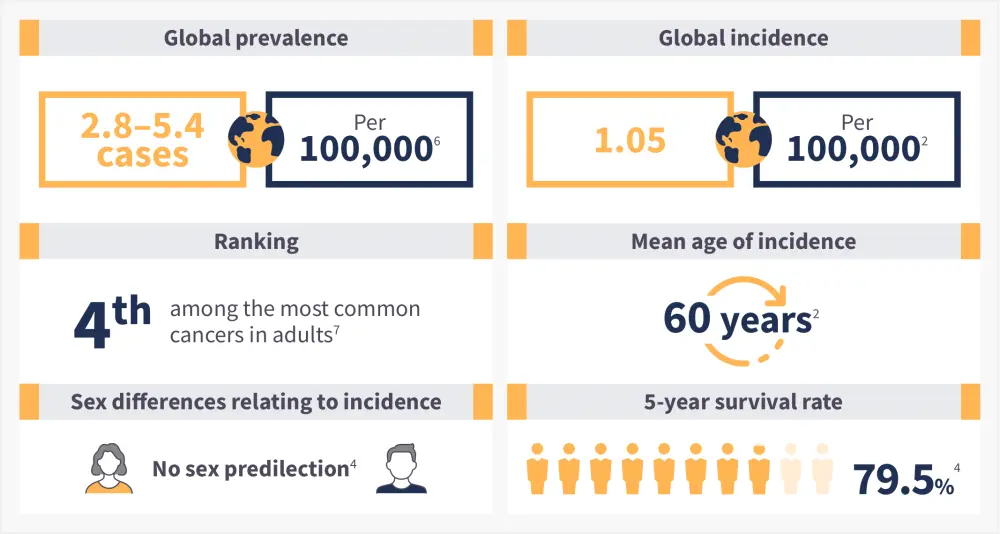
*Data from Tefferi, et al.1 and Greenfield, et al.5
Pathophysiology
It is widely accepted that a JAK2 mutation, and subsequent changes in downstream signaling, cause the recognized phenotype of increased proliferation of red blood cells, white blood cells, and platelets in PV (Figure 2).8 However, uncertainty remains around the specific interactions of erythropoietin, a key component in the development of erythrocytosis, and other associated growth factors and receptors.8
- Erythropoietin-dependent apoptosis inhibition is blocked by mutated expression of kinase-deficient dominant-negative forms of JAK2.8
- It is suggested that erythropoietin triggers the JAK/signal transducers and activators of transcription signaling pathway, inducing expression of the antiapoptotic protein B-cell lymphoma-extra large (BCL-xL).8
- BCL-xL and BCL-2 have also been found to be overexpressed on PV cells.8
- Deregulated BCL-xL expression also accounts for the continuously elevated levels of growth factor observed at all stages of the erythroid differentiation pathway.8
- PV cells exhibit hypersensitivity to antiapoptotic insulin-like growth factor-1, which in turn stimulates erythroid burst formation and overexpression of BCL-xL.
- The resulting interactions and downstream signaling lead to cellular accumulation and hypercoagulable blood, which significantly increases the risk of thrombosis.
Figure 2. Molecular pathogenesis of polycythemia vera*
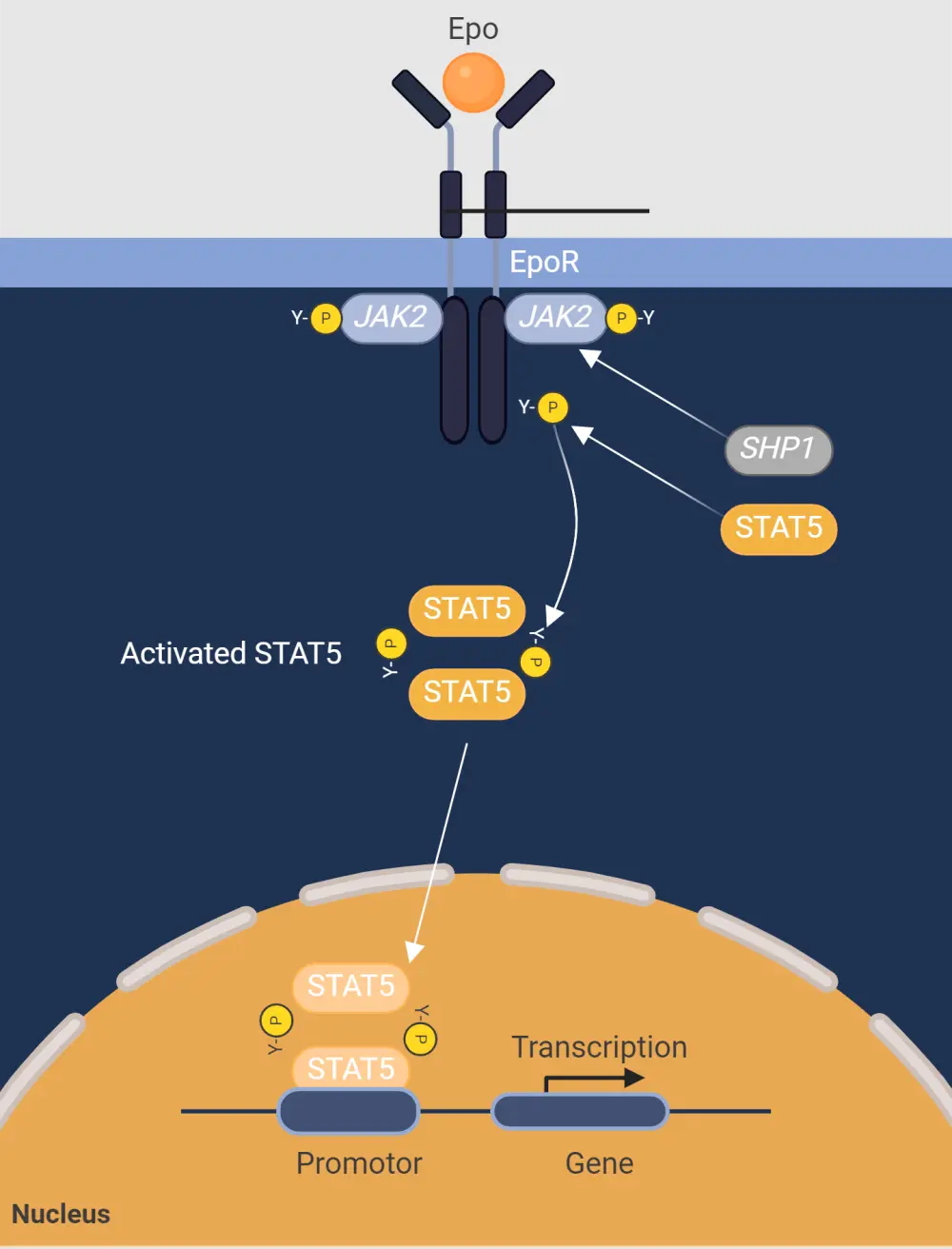
Epo, erythropoietin; EpoR, erythropoietin receptor; JAK2, Janus kinase 2; SHP1, Src homology region 2 domain containing phosphatase 1; STAT5, signal transducer and activator of transcription 5.
*Adapted from Fernandez-Luna, et al.8 Created with BioRender.com.
Signs and symptoms
- The most common complication of PV is the risk of thrombo-hemorrhagic events and transformation to acute myeloid leukemia or secondary myelofibrosis.5
- The most frequent constitutional symptoms reported by patients are fatigue, pruritus, night sweats, and bone pain (Figure 3).2
Figure 3. Most common signs and symptoms associated with polycythemia vera*
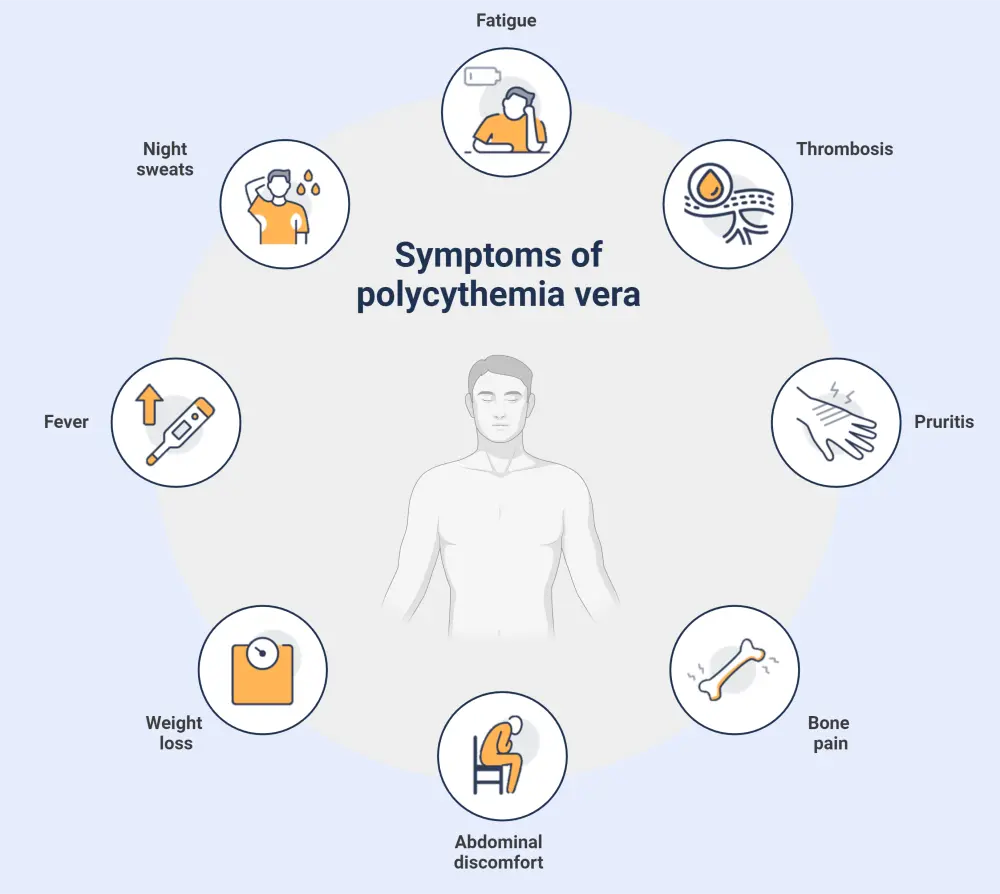
*Adapted from Khodier and Gadó.2 Created with BioRender.com.
Diagnosis
- The diagnosis of each MPN subtype has historically been defined by the World Health Organization classification of MPN.
- However, the most recent revision has resulted in a new scheme, the International Consensus Classification of MPN, with an emphasis on criteria refinement for easier distinction between subtypes.9
- For a confirmed subtype diagnosis, a patient must meet all major criteria defined in the International Consensus Classification, or most of the major criteria together with a minor criterion (Figure 4).9
Figure 4. ICC 2022 major and minor criteria for the diagnosis of PV*
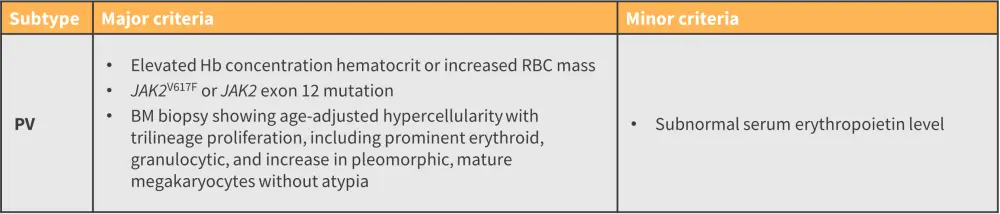
BM, bone marrow; Hb, hemoglobin; ICC, International Consensus Classification; JAK, Janus kinase; PV, polycythemia vera; RBC, red blood cell.
*Adapted from Arber, et al.9
- Once diagnosis is confirmed, conventional risk stratification is primarily based on age (>60 years/<60 years) and history of thrombosis.2
- These factors were confirmed as the most important in the multicenter European Collaboration on Low-Dose Aspirin in PV study.2
- Patients who are high risk have either or both factors present, while low-risk patients have neither factor present.2
Guidance on diagnosis may vary between countries (see key guidelines section).
Management
- Low-risk patients without any contraindications are prescribed low-dose aspirin to reduce the risk of fatal myocardial infarction, stroke, pulmonary embolism, venous thrombosis, or death from other cardiovascular causes.4
- Phlebotomy is also commonly recommended for all patients to maintain hematocrit levels of <45% in males and <42% in females.10
- Patients defined as high risk are recommended cytoreductive treatment, specifically hydroxyurea (HU).2
- Regularly, phlebotomy should be maintained alongside cytoreductive treatment.2
- Second-line therapy for patients who have become resistant or intolerant to HU is pegylated interferon (IFN) alfa-2a.2
- Pegylated IFN alfa-2a is also recommended as a first-line treatment option for patients who are pregnant or aged <40 years.2
- Although less routinely used, ruxolitinib has been investigated for use as another second-line treatment option due to the high frequency of JAK2 mutations in PV.2
- Major drug classes/types of therapy and their cellular targets are summarized in Figure 5.
Figure 5. Major types of therapy and their key cellular targets in PV*
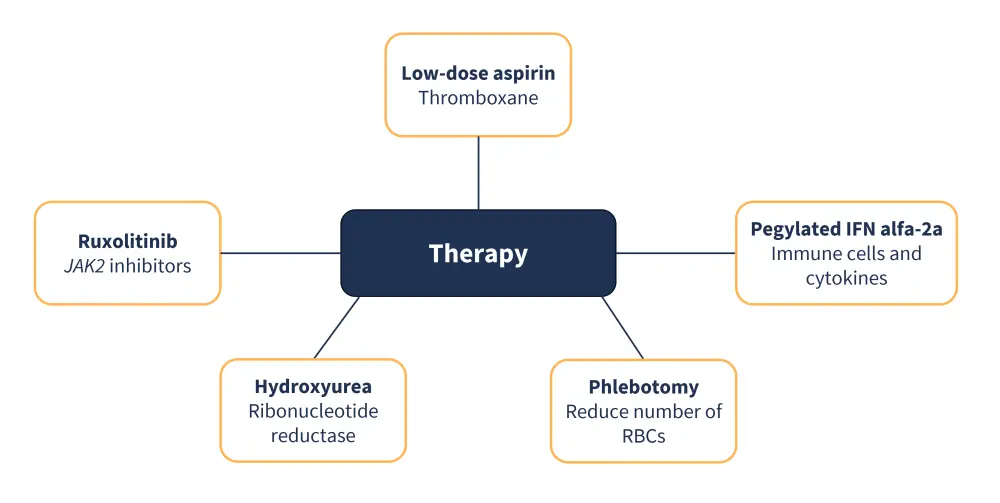
IFN, interferon; JAK2, Janus kinase 2; PV, polycythemia vera; RBC, red blood cell.
*Adapted from Spivak.10; Arif and Aggarwal.11
Treatment with aspirin is associated with a greater risk of bleeding.2
- Other more general side effects include gastrointestinal problems, ranging from gastritis to gastrointestinal bleeding.11
- HU treatment is associated with the development of anemia, leukopenia, and thrombocytopenia.2
- Treatment also increases the propensity for leukemic transformation.2
- General adverse effects associated with IFN treatment include injection-site reactions, hair and weight loss, weakness, myalgia, and severe depression.2
Guidance on management may vary between countries—see key guidelines section below.
Key Guidelines and organizations
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

