All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Ruxolitinib failure management algorithm for myelofibrosis: Canadian MPNG consensus
Myelofibrosis (MF) is a myeloproliferative neoplasm (MPN) characterized by ineffective hematopoiesis, bone marrow fibrosis and splenomegaly which carries the risk of transforming to acute myeloid leukemia (AML).1 In approximately 95% of patients the underlying MF etiology involve mutations affecting the Janus kinase (JAK)–signal transducer and activator of transcription (STAT) signaling pathway. These include driver mutations in the JAK2, calreticulin (CALR) or the thrombopoietin receptor (MPL) genes.2
The JAK inhibitor, ruxolitinib, has long provided a licensed treatment option for patients with MF improving disease-related symptoms like splenomegaly and inducing hematological responses.1,2 Nevertheless, ruxolitinib is not a curative therapy and is also associated with significant treatment-limiting side effects, including anemia, thrombocytopenia, infections and weight gain. However, there is currently limited agreement on how to define ruxolitinib failure or intolerance. These current challenges along with more management recommendations have been further discussed in our article here.
For this, the Canadian MPN Group published in the Journal of Clinical Oncology a consensus for the early recognition and management of ruxolitinib failure in MF.2 We hereby summarize the key definitions and points raised in this consensus.
1. Definition of ruxolitinib failure by the Canadian MPN Group
- Differentiation between resistance or intolerance to ruxolitinib is difficult thus, the Canadian MPN Group recommend the use of the term ‘ruxolitinib failure’ instead
- There are no good definitions of ruxolitinib failure
- Seven criteria have been defined as ruxolitinib failure by the Canadian MPN Group. These are shown in Table 1
Table 1. Canadian MPN Group criteria for defining ruxolitinib failure in MF2
|
AP, accelerated phase; BP, blast phase; Hb, hemoglobin; JAK, Janus kinase; MF, myelofibrosis; MPN, myeloproliferative neoplasm *If dose optimization does not resolve the issue consider additional treatment strategy per the management algorithms shown below |
|||
|
Pattern of ruxolitinib failure |
Definition by the Canadian MPN Group |
Expected survival |
Optimization strategy for ruxolitinib* |
|---|---|---|---|
|
Suboptimal spleen response |
< 25% reduction in palpable spleen length after at least 3 months of optimally dosed JAK inhibitor therapy |
14-18 |
Increase JAK inhibitor dose depending on Hb and platelet counts |
|
Loss of spleen response |
³ 50% increase in spleen length from best response |
14-18 |
Increase JAK inhibitor dose depending on Hb and platelet counts |
|
Transfusion-dependent anemia
|
³ 4 units of RBC transfusions in 8 weeks occurring ³ 6 months from ruxolitinib therapy |
8 |
Decrease JAK inhibitor dose |
|
Severe thrombocytopenia |
Unable to maintain unsupported platelet count > 35-50×109/L in patients receiving anticoagulation medication; and > 25×109/L in patients without coagulation |
8 |
Decrease JAK inhibitor dose |
|
Transformation to AP/BP |
|
4-6 |
Continue JAK inhibitor if required for splenomegaly and symptoms, dose adjustment dependent on Hb and platelet counts |
|
Second cancers |
|
variable |
Case-by-case discussion for JAK inhibitor discontinuation |
|
Infectious complications |
|
variable |
Consider potential benefit from adding prophylactic treatment to prevent recurrent infections |
2. Suboptimal Spleen or symptom response or loss of response
- Splenomegaly is one of the main clinical features of MF
- Suboptimal spleen response was defined by the Canadian MPN Group as a < 25% reduction in palpable spleen length from baseline or the persistence of splenomegaly
- In patients who once achieved a spleen response with ruxolitinib, loss of response was defined as > 50% increase in spleen length from the best-achieved response size
- If patients have a suboptimal spleen response or loss of response, ruxolitinib dosing should be increased if hematologically permitted
- The precise management algorithm for this type of ruxolitinib failure that is recommended by the authors is shown in Figure 1 below
Figure 1. Management algorithm for ruxolitinib failure with suboptimal or loss of spleen response2
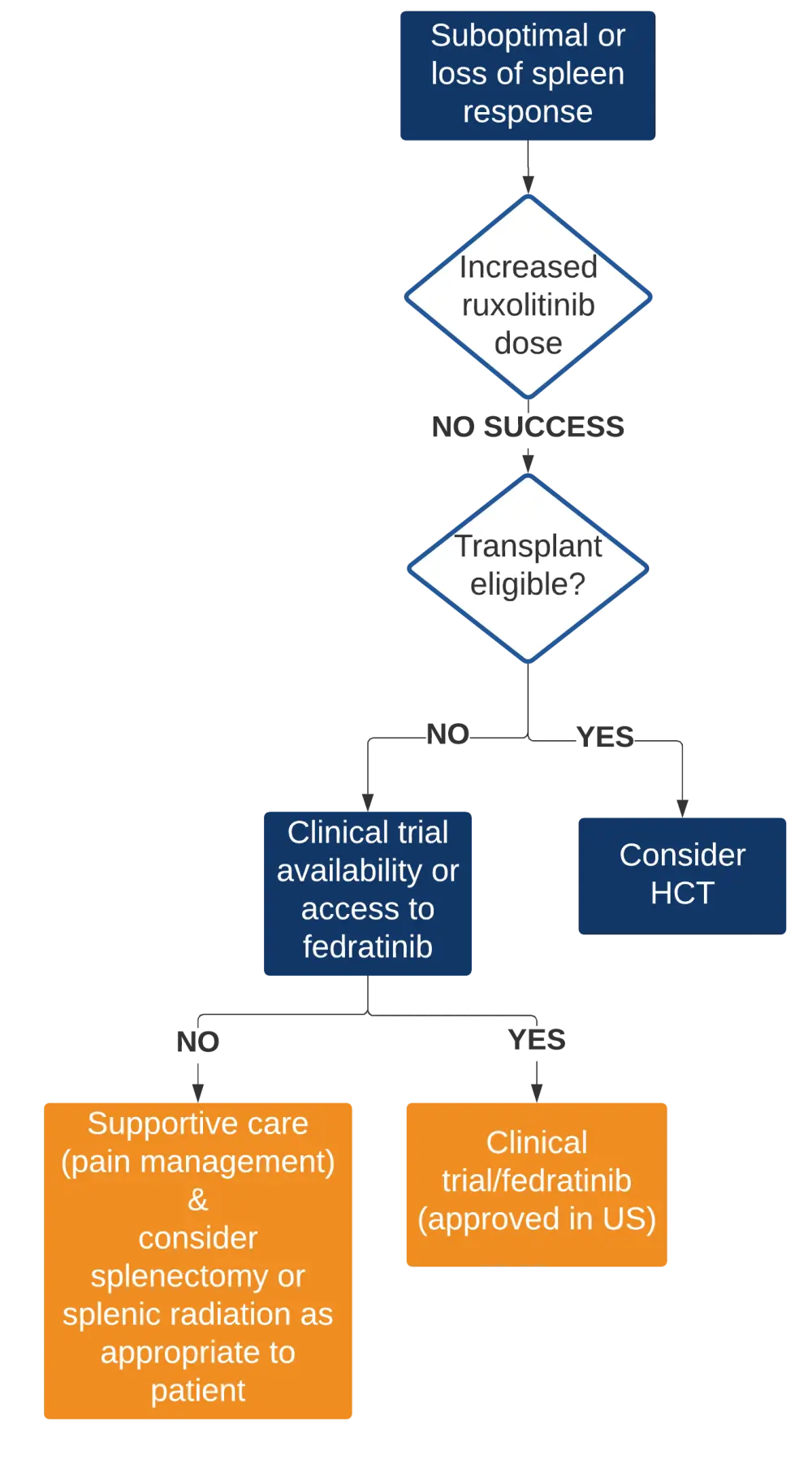
HCT, hematopoietic cell transplantation
3. Dose-limiting cytopenias
a) Anemia
- Anemia is one of the most commonly reported ruxolitinib side effects but also a clinical feature of MF
- To differentiate between the two causes, timing of anemia and other disease-related symptoms can be used:
- Ruxolitinib-induced anemia usually occurs in the first 12 weeks of treatment with a new baseline established at 24 weeks
- Ruxolitinib-induced anemia is self-limiting and dependent on the treatment dose
- Deteriorating anemia lasting longer than the first 6 months of ruxolitinib treatment, and is probably not related to ruxolitinib but a clinical MF feature
- MF-related anemia is usually accompanied by disease progression and increased frequency of disease-related symptoms, like splenomegaly
- Despite significant debate, the Canadian MPN Group reached a broader consensus regarding the potential of anemia as an indicator of ruxolitinib failure:
- Anemia which requires transfusion after 24 weeks of ruxolitinib treatment in the absence of bleeding or other causes should be considered indicative of ruxolitinib failure, in patient who were transfusion independent before treatment
- Transfusion dependence was defined by the Canadian MPN group as the need of minimum 4 units of packed red blood cells during a period of 8 weeks and without the occurrence of active bleeding or other causes
- The authors mentioned that erythropoietin-stimulating agents (ESAs) should be considered in transfusion-dependent patients who are generally doing well on ruxolitinib
- The management algorithm for patients with severe anemia is shown below in Figure 2
Figure 2. Management algorithm for ruxolitinib failure with a new onset transfusion-dependent anemia2
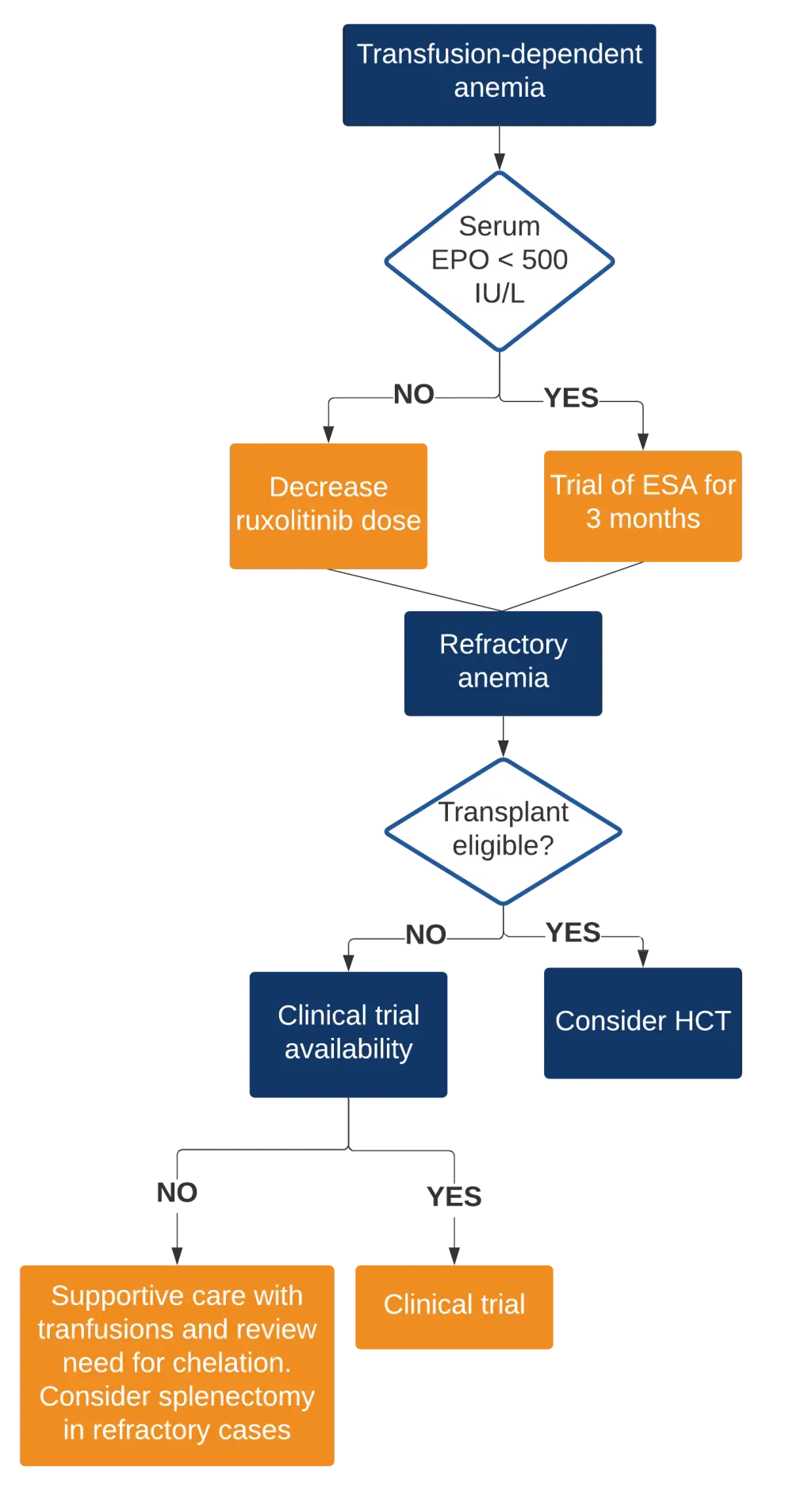
EPO, erythropoietin; ESA, erythropoietin-stimulating agent; HCT, hematopoietic cell transplantation
b) Thrombocytopenia
- Thrombocytopenia is another dose-dependent side effect of ruxolitinib
- Patients with MF and severe thrombocytopenia have higher disease burden, poorer outcomes, and are difficult to manage
- Hematopoietic cell transplantation (HCT) should be considered early in this population
- If severe thrombocytopenia occurs while a patient is on a stable ruxolitinib dose, it is suggestive of disease progression and poor outcome with an estimated survival between 6-9 months
- The proposed management algorithm for ruxolitinib failure with severe thrombocytopenia is shown in Figure 3
Figure 3. Management algorithm for the ruxolitinib failure with severe thrombocytopenia2
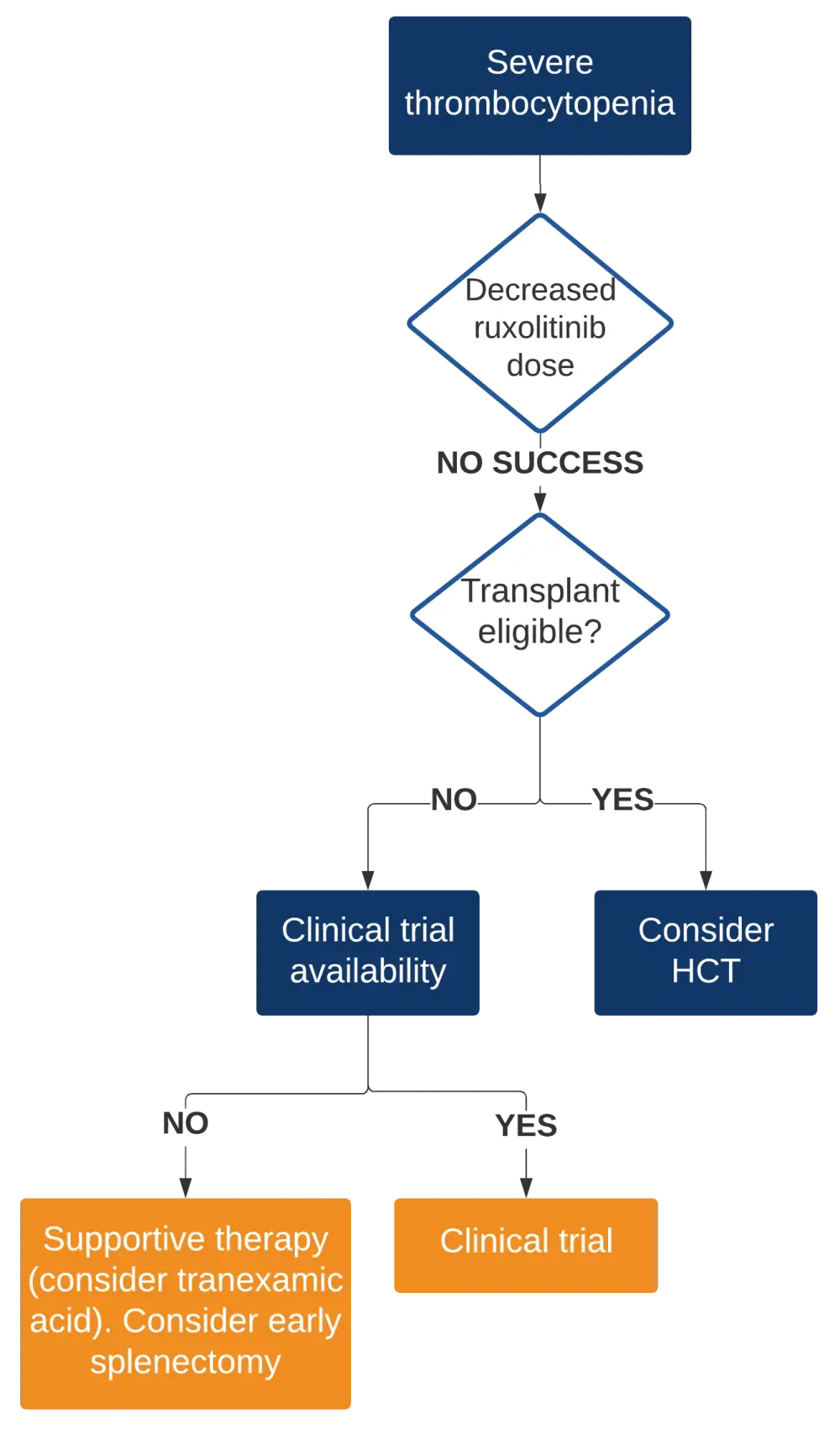
HCT, hematopoietic cell transplantation
4. Transformation to accelerated or blast phase
- No consensus was reached for the definition of the magnitude of change in peripheral blood blasts that indicate the need for treatment re-evaluation
- The authors suggested that the transformation to both accelerated phase (AP) or blast phase (BP) should be considered as ruxolitinib treatment failure and that these two patient populations should be treated similarly according to the management algorithm shown in Figure 4
Figure 4. Management algorithm for the ruxolitinib failure with severe thrombocytopenia2
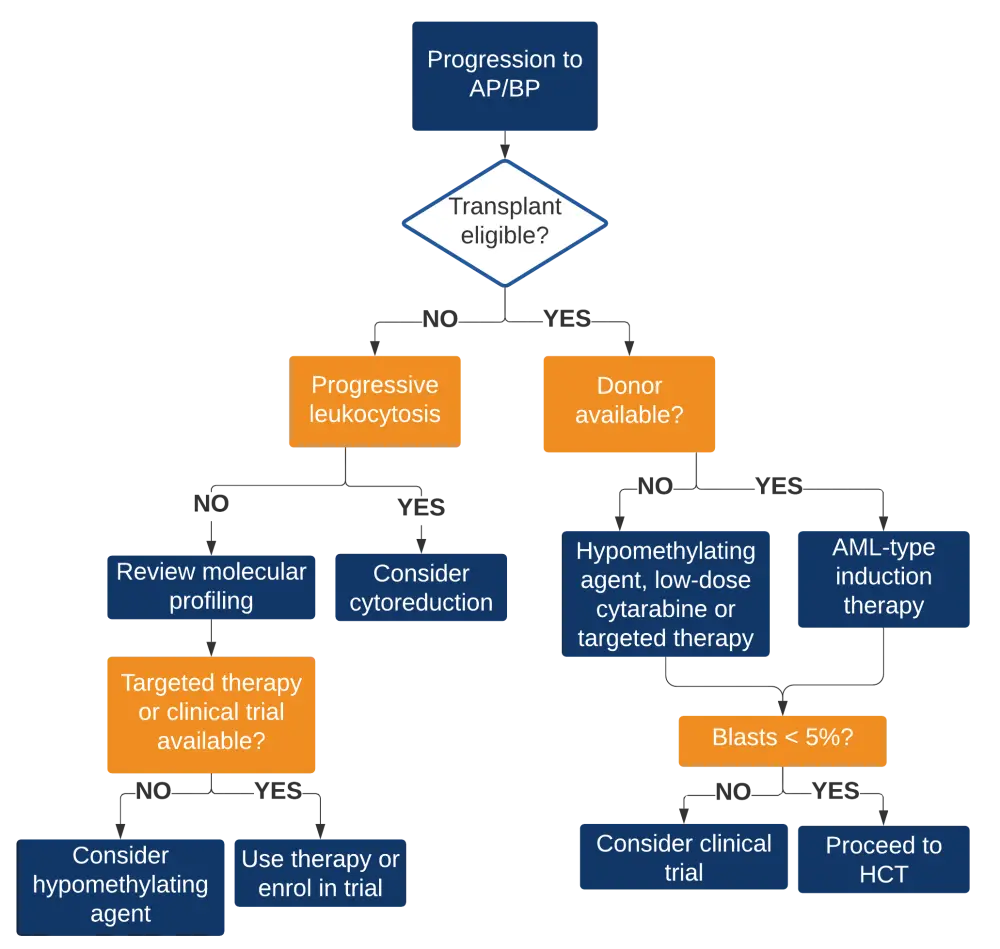
AP, accelerated phase; AML, acute myeloid leukemia; BP, blast phase; HCT, hematopoietic cell transplantation
5. Secondary cancers
- The occurrence of secondary cancers during ruxolitinib treatment is likely due to the immunosuppressive effects of the regimen due to its interference with the JAK/STAT pathway that is responsible for the production of many cytokines
- The Canadian MPN Group recommends that ruxolitinib treatment is used continuously in patients that develop localized basal or squamous cell skin carcinoma, unless it is recurrent or aggressive
- Moreover, clinically aggressive secondary cancers should be discussed for each individual case with medical oncologists to determine if ruxolitinib should be discontinued
6. Recurrent infections
- There is a higher risk of opportunistic infections while being treated with ruxolitinib due to the inhibition of JAK1-mediated cytokine production and dendritic cell function impairment
- Herpes zoster, urinary tract infections and bronchitis seem to be common ruxolitinib-associated infections
- There are no guidelines concerning the use of prophylactic agents to prevent infections in MF patients receiving ruxolitinib
- The authors recommend the following
- Treatment of active infections should be followed by prophylactic treatment to prevent secondary infections
- Before starting treatment with ruxolitinib, patients should be screened for hepatitis and HIV. Screening for tuberculosis should be considered for patients from endemic regions
- Ruxolitinib treatment should be continued in case of common infections like herpes zoster or urinary tract infections
- Life-threatening infections need careful risk-benefit analysis on an individual basis
7. Switching from ruxolitinib to alternative therapy
- Patients requiring alternative treatments like HCT, participation in another clinical trial or second-line JAK inhibitors will need to discontinue ruxolitinib
- Sudden ruxolitinib discontinuation may lead to withdrawal symptoms, thus the recommendations are as follow
- For HCT patients: slow taper over 5 days and the last dose of ruxolitinib should be taken 1 day before conditioning therapy
- For other patients: dose decrease per week by 5 mg twice daily with close symptom monitoring
- A short course of steroids may be helpful until the start of a new regimen
Conclusions
The Canadian MPN group have provided useful guidelines and management algorithms for the identification of MF patients with ruxolitinib failure. Recognition of the exact pattern of failure following ruxolitinib treatment is crucial for the management and outcomes of patients. A summary of the key recommendations discussed above are presented as useful practice points in Table 2 below.
Table 2. Useful practice points by the Canadian MPN Group for the management of MF patient with ruxolitinib failure2
|
DIPSS, Dynamic International Prognostic Stratification System; HCT, hematopoietic cell transplantation; MF, myelofibrosis; RBC, red blood cell |
|
|
1 |
Ruxolitinib dose optimization should be attempted in MF patients with suboptimal response or those who develop severe cytopenias |
|---|---|
|
2 |
Patient survival after ruxolitinib failure is poor and variable depending on the cause (see Table 1). Patients should be clinically evaluated by experts if ruxolitinib failure is suspected |
|
3 |
HCT should be considered in eligible patients who failed ruxolitinib treatment. Consider earlier HCT referral in high-risk patients for ruxolitinib failure (i.e. high-risk DIPSS, RBC transfusion-dependent prior ruxolitinib, high-risk mutations like ASXL1 and EZH2) |
|
4 |
Patients not eligible for transplant should be offered enrolment in clinical trials |
|
5 |
When switching patients from ruxolitinib to alternative therapy, gradual taper should be considered, as sudden discontinuation can lead to withdrawal symptoms |
|
6 |
For symptomatic refractory splenomegaly, transfusion-dependent anemia and severe thrombocytopenia, symptom control such as splenectomy is a useful option |
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

