All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Selinexor granted orphan drug designation by the European Commission for the treatment myelofibrosis
On November 1, 2022, the European Commission granted orphan drug designation to selinexor for the treatment of patients with myelofibrosis.1 Earlier this year, on May 26, 2022, the U.S. Food and Drug Administration (FDA) granted the same designation.
Selinexor is a first-in-class oral exportin 1 (XPO1) inhibitor; inhibition of the exportin protein stops tumor suppressor, growth regulatory, and anti-inflammatory proteins being transported from the cell nucleus to the cell cytoplasm (Figure 1).1 The subsequent buildup of these proteins within the cell nucleus results in anticancer activity, as well as a counteraction of any active oncogenic signaling.1 There are currently three ongoing clinical trials investigating selinexor in myelofibrosis, which we summarize below.
Figure 1. Selinexor mechanism of action*
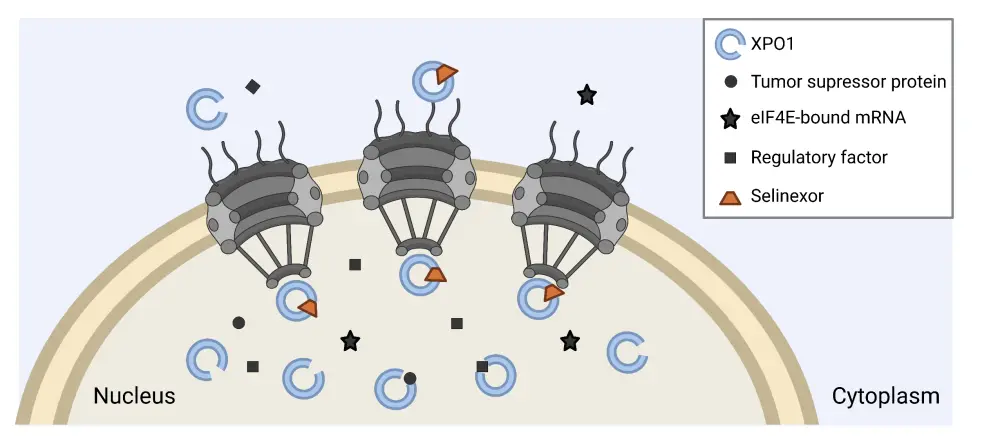
eIF4E, eukaryotic translation initiation factor 4E; XPO1, exportin 1.
*Adapted from Karyopharm Theraputics.2 Created with BioRender.com.
Ongoing clinical trials3
Selinexor monotherapy
The ESSENTIAL trial (NCT03627403) is a single-arm, phase II study evaluating selinexor monotherapy for patients diagnosed with primary myelofibrosis, post-essential thrombocytosis, or post-polycythemia vera who have had previous exposure to ruxolitinib or another JAK inhibitor. Prior to study protocol version 7, patients were orally administered 60 mg or 80 mg selinexor once a week until disease progression, toxicity, or no sign of clinical benefit. Any patients enrolled after protocol version 7 will be administered selinexor orally, beginning at 40 mg once per week. The primary endpoint of the study has been set at changes in spleen volume. Secondary endpoints are changes in symptom score, overall survival, overall response rate, and safety. Data from the first 12 patients enrolled in this trial were presented at the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition in 2021; check out this previous MPN Hub article for a summary.
Selinexor versus clinician’s treatment choice
A randomized phase II trial (NCT04562870) is investigating the safety and efficacy of selinexor compared with the treatment choice of a clinician in patients diagnosed with myelofibrosis who have had ≥6 months prior treatment with a JAK inhibitor. The primary endpoint of the study has been set as the percentage of patients with a reduction in spleen volume ≥35%. Secondary endpoints include overall survival, overall response rate, and the percentage of patients with a reduction in total symptom score ≥50%.4 This trial is estimated to enroll a total of 112 patients.4
Selinexor in combination with ruxolitinib
A global phase I/II study (NCT04562389) is investigating selinexor in combination with ruxolitinib for the treatment of patients diagnosed with myelofibrosis who have received no prior related therapy. The phase Ia section of the study aims to establish the recommended phase II dosing, as well as generate initial safety and efficacy data through a 3 + 3 trial design. The phase Ib section of the study will further evaluate the recommended phase II dosing and provide additional safety and efficacy findings. The full trial design is shown in Figure 2. During the phase II part of the study, patients will be randomly assigned either the combination therapy or ruxolitinib alone. Data from this trial is set to be presented at the 64th ASH Annual Meeting and Exposition on December 10–13, 2022, in New Orleans, US.5
Figure 2. NCT04562389 trial design*
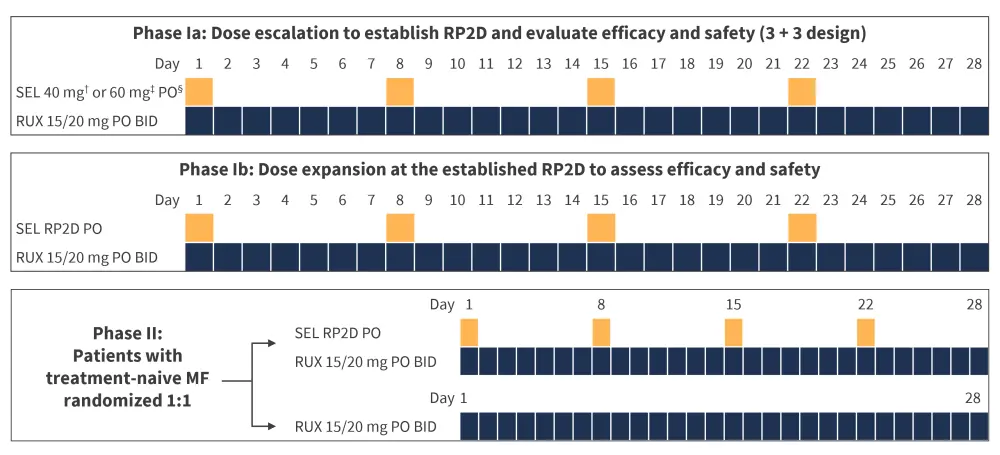
BID, twice a day; MF, myelofibrosis; PO, orally; RP2D, recommended phase II dose; RUX, ruxolitinib; SEL, selinexor.
*Adapted from ClinicalTrials.gov.6
†Cohort 1 received SEL 40 mg PO.
‡Cohort 2 received SEL 60 mg PO.
§Cohort −1 received SEL 20 mg PO twice weekly.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

