All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Targeting PI3K pathway with parsaclisib when ruxolitinib response is not optimal in the treatment of myelofibrosis: Results from a phase II trial
Ruxolitinib, a Janus kinase (JAK) 1/2 inhibitor, has been associated with improved clinical symptoms and reduced spleen size in the treatment of myelofibrosis (MF). As JAK pathway may not be the only pathway involved in the pathogenesis of myeloproliferative neoplasms (MPN), some patients may not achieve optimal response with ruxolitinib therapy. Phosphatidylinositol 3 kinase (PI3K)/AKT/mTOR is another signaling pathway, which when hyperactivated or deregulated, has shown to be associated with different cancer types or hematological malignancies. Previous studies have demonstrated the importance of activation of PI3K pathway in MPN which could be targeted therapeutically.1,2
In a phase II study (NCT02718300), Yacoub and colleagues investigated whether adding parsaclisib, a potent, highly selective next generation PI3Kδ inhibitor, to ruxolitinib would improve outcomes in patients with suboptimal response to ruxolitinib therapy. They presented their results during the virtual edition of the 25th European Hematology Association (EHA) Annual Congress, and data are summarized below.2
Study design2
- Eligibility criteria:
- Primary or secondary MF (to polycythemia vera or essential thrombocythemia) with suboptimal response to ruxolitinib therapy
- Aged ≥ 18 years
- Platelet count ≥ 50 × 109/L within 4 weeks prior to screening
- Suboptimal response was defined as
-
- ruxolitinib treatment ≥ 6 months with stable dose for at least 8 weeks before study entry (at a dose of 5–25 mg twice daily) AND
- palpable spleen size > 10 cm below left subcostal margin OR
- palpable spleen size between 5–10 cm below left subcostal margin with active MF symptoms (one symptom score ≥ 5 or two symptom scores ≥ 3 each
- Primary endpoint: Change in spleen volume from baseline to Week 12 by MRI or CT scan
- Secondary endpoints: Change in spleen length and symptoms on the MF Symptom Assessment Form Total Symptom Score (MFSAF-TSS), and safety
As of cutoff date, a total of 53 patients were enrolled (n = 10 for safety run-in; n = 43 for randomization and dose expansion). Patients continued their ruxolitinib therapy and were randomized into two different dosing regimens:
- Daily/weekly (QD/QW) dosing group (n = 33): Ruxolitinib + 10 mg/20 mg parsaclisib QD for 8 weeks, and ruxolitinib + same QD dose of parsaclisib QW thereafter
- All daily (QD) dosing group (n = 20): Ruxolitinib + 5 mg/20 mg parsaclisib QD for 8 weeks, and ruxolitinib + 5 mg parsaclisib QD thereafter
Patient characteristics2
Baseline characteristics were well balanced between dosing groups (see Table 1). The median age was 66 years (range, 41–89) and similar in both groups. All patients, except one in the QD/QW group, had palpable spleen with a median spleen length of 13.5 cm (range, 5–30).
Table 1. Patient characteristics2
|
MPN-SAF, myeloproliferative neoplasm symptom assessment form; PET-MF, post-essential thrombocythemia myelofibrosis; PMF, primary myelofibrosis; PPV-MF, post-polycythemia vera myelofibrosis; QD, once daily; QW, once weekly; TSS, total symptom score |
||
|
Characteristic |
QD/QW dosing (n = 33) |
QD dosing (n = 20) |
|---|---|---|
|
Median time since diagnosis, months (range) |
31.2 (6.7–268.9) |
30.4 (4.9–98.5) |
|
Median duration of previous ruxolitinib use, months (range) |
18 (6–94) |
19 (4.8–56) |
|
Median volume of spleen, cm3 (range) |
2,333 (327–5,324) |
1,890 (434–3,741) |
|
Median TSS score by MPN-SAF (range) |
24 (0–69) |
27 (3–65) |
|
MF subtype, n (%) PMF PPV-MF PET-MF |
17 (52) 12 (36) 4 (12) |
10 (50) 8 (40) 2 (10) |
Results2
Treatment discontinuation rates were 82% and 40% in the QD/QW and QD groups, respectively, and were mainly due to disease progression or lack of efficacy. Median duration of treatment was 48.4 (7.3–123.8) weeks and 22.4 (4.6–60.1) weeks with QD/QW dosing and QD dosing, respectively.
Spleen volume
The responses were classified into three categories, reduction by ≥ 10%, ≥ 25%, and ≥ 35%, and measured at Weeks 12 and 24. Results from both time points are shown in Figure 1 and 2, respectively. Median percentage change at Week 12 and Week 24 with QD/QW dosing and QD dosing were −2.3 and −15.4, and −2.5 and −25.4, respectively. These results indicate that the treatment effect was more profound at Week 24 and in favor of QD dosing at both timepoints.
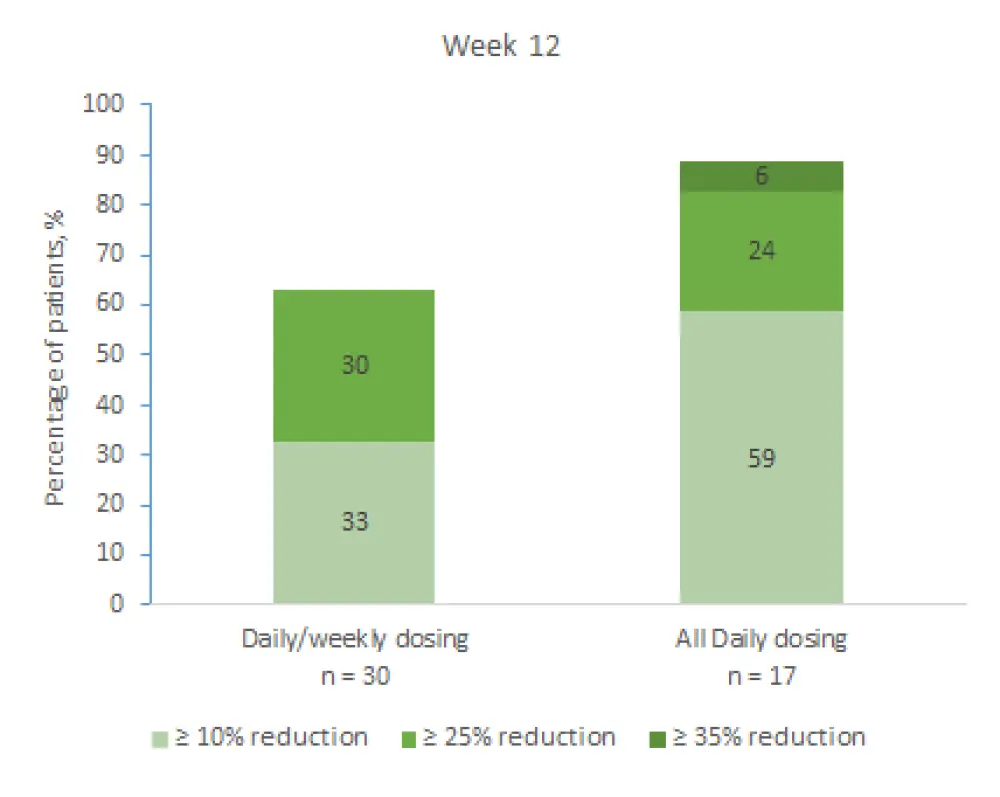
Figure 1. Change in spleen size at Week 12
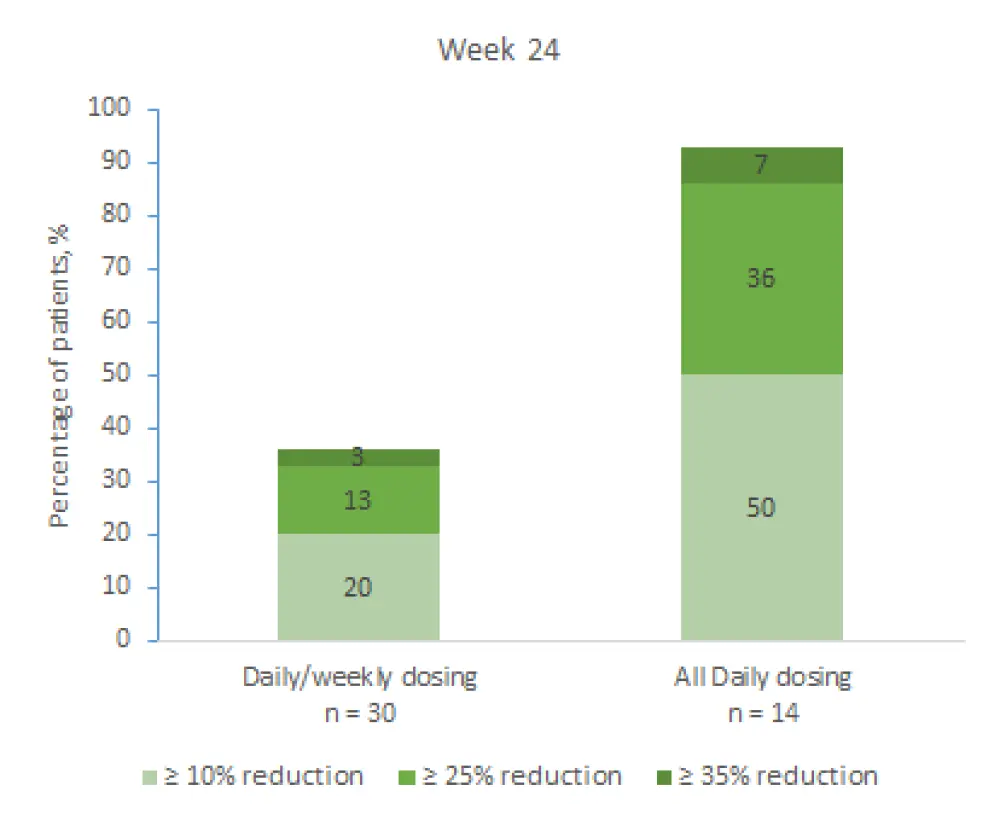
Figure 2. Change in spleen size at Week 24
Spleen length
The reduction in spleen length was continuous up to 24 weeks with QD/QW dosing and 5 mg QD dosing (the lowest dose used in the trial), with a greater mean change with 5 mg QD dosing.
MFSAF-TSS
At Week 4, ≥ 50% improvement in the total symptom score was observed in 19% and 18% of patients in the QD/QW and QD dosing, respectively, and continued up to 24 weeks. At Week 12, median percentage change was greater with QD dosing (−39.6 vs −14.0 with QD/QW dosing).
Safety2
The number of most common (occurred in ≥ 15%) Grade ≥ 3 nonhematologic treatment-emergent adverse events (TEAEs) was very low and mostly seen in the QD/QW dosing group, and included diarrhea (3%, QD/QW), nausea (3%, QD/QW), fatigue (3% in QD/QW, and 5% in QD), fall (6%, QD/QW), back pain (3%, QD/QW) and dyspnea (5%, QD). Urinary tract infection (n = 3), pneumonia, pyrexia, and fall (n = 2 each) were serious TEAEs (occurred in ≥ 2%). No events of colitis, cytomegalovirus reactivation, Pneumocystis jiroveci pneumonia or pneumonitis occurred in both arms, and elevated values in the liver function test (6%), Grade ≥ 2 rash (3%), and diarrhea (12%) were observed with QD/QW dosing. Two TEAEs of varicella-zoster virus infection were reported (n = 1 each arm).
In terms of hematologic TEAEs:
- New-onset Grade 3 thrombocytopenia was reported in 18% of patients receiving QD/QW dosing and in 30% of those receiving QD dosing
- New-onset Grade 4 thrombocytopenia was reported in 21% of patients receiving QD/QW, while there were no reports in the QD group
There were no significant changes in hemoglobin levels in either treatment group
The number of deaths was seven (acute myeloid leukemia blast crisis and pneumonia, n = 2 each) and none of them were found to be related to parsaclisib or ruxolitinib. TEAEs leading to discontinuation were thrombocytopenia, fatigue, blast crisis, disseminated tuberculosis, pathological fracture events in the QD/QW dosing group, and only one case of leukocytosis in the QD dosing group.
Conclusion
These data indicate that adding parsaclisib treatment regimen in patients with suboptimal response to stable ruxolitinib doses was beneficial for rapid and durable reduction in spleen volume and improvement of symptoms. The combination was well tolerated with low rates of Grade ≥ 3 adverse events, and few common side effects associated with PI3Kδ inhibitors such as hepatic events, rash, and colitis. All daily dosing scheme with parsaclisib appeared to be more effective in reducing spleen volume and length, improving symptom burden, and was associated with a more favorable safety profile compared with daily/weekly dosing. Further studies are needed to support these results.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

