All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
KRT-232-109: Navtemadlin plus ruxolitinib in MF with a suboptimal ruxolitinib response
Myelofibrosis (MF) is a myeloproliferative neoplasm characterized by splenomegaly and significant symptom burden.1 Although the Janus kinase inhibitor ruxolitinib has improved splenomegaly and MF-related symptoms in patients with MF, the majority achieve suboptimal responses.1
Navtemadlin is a potent, selective, oral, mouse double minute 2 inhibitor (MDM2i) that restores p53 activity and modulates the expression of pro-apoptotic Bcl-2 family proteins to induce apoptosis of TP53wt CD34+ myeloblasts.1 In a phase II study, single-agent navtemadlin demonstrated promising clinical and disease-modifying activity in patients with relapsed/refractory MF. Preclinical studies have demonstrated the synergistic effects of navtemadlin plus ruxolitinib in mediating p53-dependent apoptosis of myeloblasts by inhibiting checkpoint p21-mediated cell-cycle arrest, providing the rationale for investigation of the combination in the clinical setting.1
Below, we summarize one of our European Hematology Association (EHA) 2023 top abstracts reporting the efficacy and safety of navtemadlin combined with ruxolitinib in patients with primary or secondary MF who have a suboptimal response to ruxolitinib, presented by John Mascarenhas.1
Study design
KRT-232-109 (NCT04485260), is a global, open-label, multicenter, phase IIb/II study, which included patients with:
- Primary, post-polycythemia vera, or post-essential thrombocythemia MF
- Suboptimal response to ruxolitinib after ≥18 weeks
- No evidence of response or progression on ruxolitinib
- Platelet count >100 × 109/L
- Wild-type p53
The phase I dose escalation evaluated three doses of navtemadlin (120 mg, 180 mg, and 240 mg) added to a pre-study ruxolitinib dose (≥8 weeks) to identify the recommended phase II expansion dose (RP2D). In the phase II dose expansion, navtemadlin at 240 mg was administered for 7 days, with 21 days off in a 28-day cycle.
Primary endpoints:
- To determine the recommended phase II dose (RP2D) of navtemadlin at phase I
- Spleen response rate at Week 24, defined as spleen volume reduction by central review at phase II
Key secondary endpoints:
- Symptom improvement from ≥50% at Week 24
- Spleen response duration
- Red blood cell transfusion independence
- Spleen response rate at any time point from baseline
Results
Overall, 28 patients were treated with navtemadlin at the RP2D of 240 mg. Baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
DIPPS, Dynamic International Prognostic Scoring System; MF, myelofibrosis; TSS, total symptom score. |
|
|
Characteristic, % (unless otherwise stated) |
Navtemadlin 240 mg + ruxolitinib |
|---|---|
|
Median age at diagnosis (range), years |
63 (40–85) |
|
DIPPS |
27 |
|
Intermediate 1 |
43 |
|
Intermediate 2 |
32 |
|
High |
25 |
|
MF subtype |
|
|
Primary |
36 |
|
Secondary |
64 |
|
Median spleen volume at baseline (range), cm3 |
2,039 (650–3,549) |
|
Median platelets (range), 106/L |
165 (100–636) |
|
Transfusion dependence, n |
25 |
|
Median prior lines of therapy (range), n |
1 (1–5) |
|
Median TSS (range) |
15 (3.2–49.1) |
|
Median duration of prior ruxolitinib therapy (range), months |
21.6 (7–129) |
|
Ruxolitinib dose at entry† |
|
|
5 mg |
14 |
|
10 mg |
25 |
|
15 mg |
18 |
|
20–25 mg |
39 |
Efficacy
Among evaluable patients (n = 19) at the May 2, 2023, data cut off:
- Spleen volume reduction of 35% and 25% at Week 24 was achieved in 42% and 32% of patients, respectively.
- Total symptom score improvements of ≥50% was attained in 32% of patients at Week 24.
- The median time on treatment was 6 months and ongoing.
- There was a reduction in the CD34+ myeloblasts from baseline until Week 24.
Among seven evaluable patients, 71% achieved a ≥20% reduction of driver VAF, and 57% had improved bone marrow fibrosis of Grade >1 by central review.
Safety
Overall, treatment emergent adverse events (TEAEs) were mostly Grade 1–2. Any grade and Grade 3–4 TEAEs were reported in 96% and 46% of patients, respectively.
- The most common gastrointestinal toxicities were Grade 1–2 nausea, diarrhea, and vomiting.
- These were mostly seen in the first two cycles and within the 7-day treatment period.
- Grade 3/4 TEAEs included thrombocytopenia (29%), anemia (18%), nausea (7%), diarrhea (4%), vomiting (4%), and asthenia (4%).
- The most common any grade TEAEs were nausea, diarrhea, thrombocytopenia, constipation, vomiting, asthenia, anemia, and fatigue (Figure 1).
- There was no progressive decline in the mean platelet and hemoglobin count over time.
Figure 1. Any grade TEAEs in ≥10%*
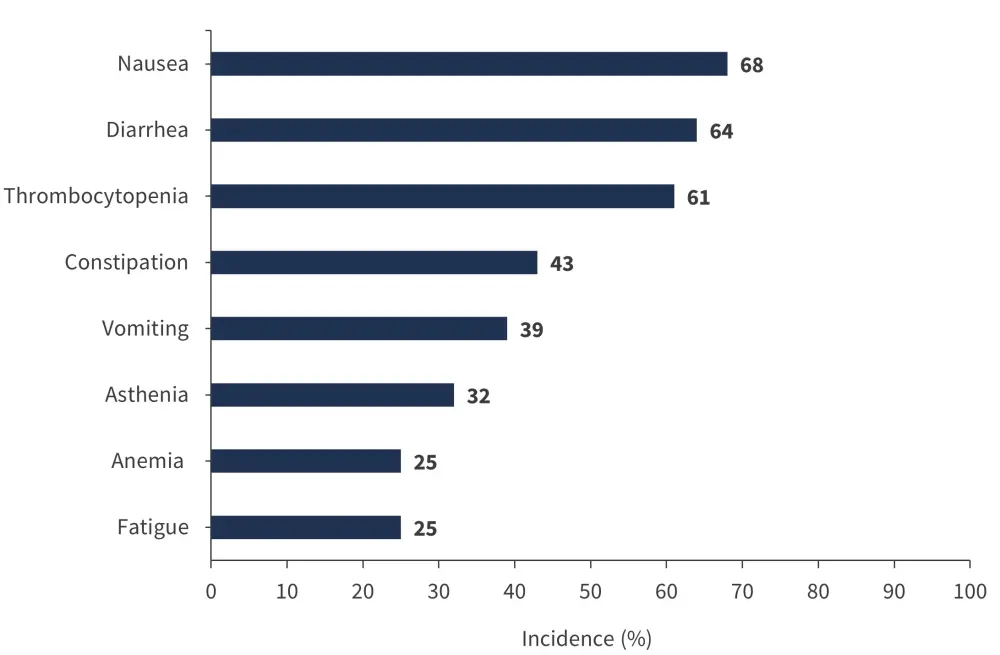
*Data from Mascarenhas.1
Presenter’s conclusion
This phase II study demonstrated the clinically meaningful benefit and safety of navtemadlin plus ruxolitinib in patients with primary or secondary MF who had a suboptimal response to ruxolitinib. This combination resulted in reduced spleen volume, reduced symptom burden, reduced percentage of CD34+ cells, as well as driver VAF and bone marrow fibrosis reductions, with low incidences of high-grade adverse events. These data support the evaluation of this novel combination in a phase III study.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


