All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Momelotinib long-term safety: Pooled analysis from MOMENTUM, SIMPLIFY-1, and SIMPLIFY-2
Myelofibrosis (MF) is commonly characterized by splenomegaly, progressively worsening anemia, and thrombocytopenia.1 Treatment options currently include the Janus kinase (JAK) inhibitors ruxolitinib and fedratinib, which are approved globally, as well as pacritinib, which is approved by the U.S. Food and Drug Administration (FDA).1 Despite the clinical benefit of these drugs, their respective toxicity profiles and other factors may limit their use.
To address this, momelotinib, a first-in-class oral inhibitor of activin A receptor type 1, JAK1, and JAK2 is currently under investigation, and has demonstrated clinical activity in several phase III randomized trials: MOMENTUM (NCT04173494), SIMPLIFY-1 (NCT01969838), and SIMPLIFY-2 (NCT02101268).1 Verstovsek et al.1 conducted a pooled data analysis from these three trials in order to evaluate the safety and tolerability of momelotinib in patients diagnosed with MF. We summarize their key findings here.
Study design
- Data were pooled from patients enrolled in MOMENTUM, SIMPLIFY-1, and SIMPLIFY-2 diagnosed with intermediate- and high-risk MF and having received ≥1 dose of momelotinib.
- Adverse events (AEs) were defined as those that started or worsened after the first dose of momelotinib or ≤30 days after the last dose.
- AEs of clinical importance were defined as all infections, malignancies, major cardiovascular events, anemia, neutropenia, thrombocytopenia, peripheral neuropathy, thromboembolism, and hemorrhage.
- The incidence of AEs was analyzed at Week 24 and Week 48 of treatment.
Results
- A total of 725 patients were included in the analysis.
- The median duration of momelotinib exposure was 11.3 months.
- 12.1% of patients continued treatment for ≥5 years.
- 50.6% of patients received ≥48 weeks of momelotinib treatment.
- 13.8% of patients discontinued treatment due to MF progression, followed by 4% due to infection, and 3.7% due to thrombocytopenia.
Full patient characteristics are shown in Table 1.
Table 1. Patient characteristics*
|
DIPSS, Dynamic International Prognostic Scoring System, JAK. Janus kinase. |
|
|
Characteristic, % (unless otherwise stated) |
Pooled momelotinib treated patients |
|---|---|
|
Mean age, years |
66.4 |
|
Race |
|
|
White |
81.7 |
|
Black |
1.8 |
|
Asian |
7.3 |
|
Other |
1.9 |
|
Not reported |
7.3 |
|
DIPSS risk category |
|
|
Intermediate-1 |
17.0 |
|
Intermediate-2 |
41.4 |
|
High |
41.5 |
|
Not reported |
0.1 |
|
Hemoglobin ≥8 g/dL |
81.0 |
|
Mean hemoglobin, g/dL |
9.9 |
|
Prior JAK inhibitor treatment |
43.3 |
|
Transfusion independent |
49.5 |
|
Transfusion dependent |
34.9 |
|
Mean platelet count, × 109/L |
238.9 |
The most common nonhematologic AEs of any grade are shown in Figure 1.
Figure 1. Most common nonhematologic adverse events of any grade*
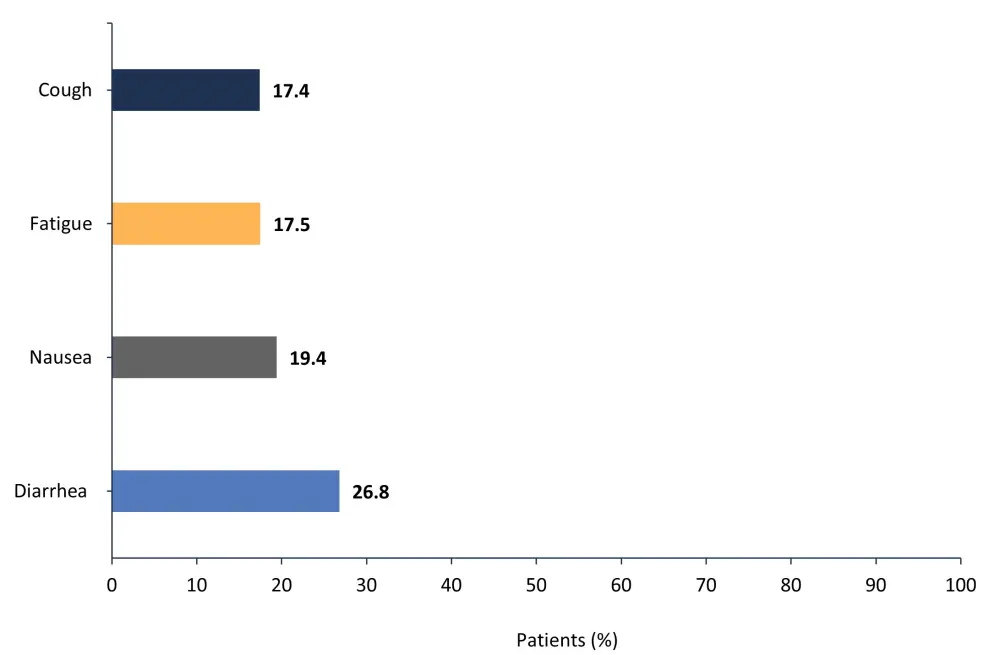
*Adapted from Verstovsek, et al.1
- Most gastrointestinal AEs were Grade 1–2.
- Peripheral sensory neuropathy was observed in 12.3% of patients.
- Grade ≥3 peripheral sensory neuropathy was seen in 0.7% of patients.
- The most common Grade ≥3 nonhematologic AE was pneumonia (8.4% of patients).
- The most common hematologic AEs of any grade and Grade ≥3 are shown in Figure 2.
Figure 2. Most common hematologic adverse events of any grade and Grade ≥3*
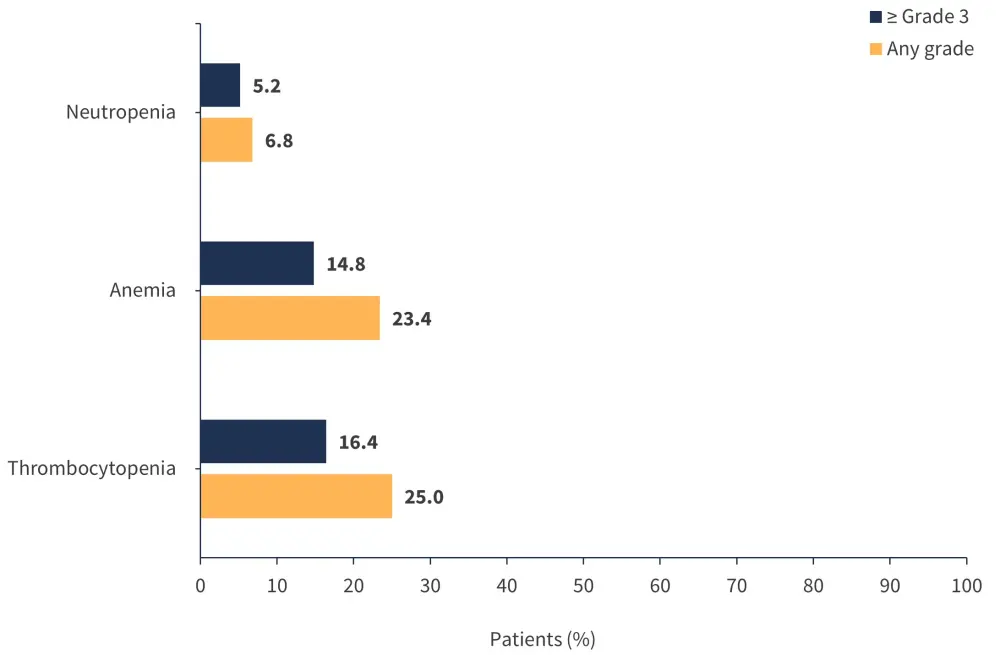
*Adapted from Verstovsek, et al.1
Serious hematologic AEs were seen in <5% of patients.
AEs of clinical importance
- No new AEs were reported.
- Most AEs were experienced in the first 24 weeks of treatment.
- Infection was the most common AE in patients (55.4%).
- 61.7% of patients had Grade 1–2 infections.
- The incidence of infections did not increase over time.
- Opportunistic infections occurred in 5.5% of patients.
- The incidence over time remained stable.
- Serious infections occurred in 0.8% of patients and none were fatal.
- Malignancies of any grade were reported in 13.4% of patients.
- Grade ≥3 malignancies were reported in 7.3% of patients.
- Rates of malignancies were within the expected range for the patient age group.
- The rate of incidence did not increase over time.
- Non-melanoma skin cancer was reported in 4.8% of patients.
- Most cases were Grade 2 and none were fatal or caused treatment discontinuation.
- Acute myeloid leukemia was observed in 3% of patients.
- Most cases were in the first 24 weeks of treatment.
- There was no increase in incidence.
- Major cardiovascular events were reported in 7.9% of patients.
- Grade ≥3 events occurred in 6.6% of patients.
- 1.5% of cases were fatal, but there was no consistent trend towards increased incidence.
- Thromboembolism was reported in 8.8% of patients.
- Grade ≥3 thromboembolism occurred in 5.4% of patients.
- The rate of incidence did not increase.
- Hemorrhage was reported in 28.6% of patients.
- Most cases were Grade 1–2.
- 32.2% of patients with a platelet count <150 × 109/L reported a hemorrhage event.
- Peripheral neuropathy was seen in 14.8% of patients.
- Peripheral sensory neuropathy was reported in 12.3% of patients.
- Peripheral sensorimotor neuropathy was seen in 0.7% of patients.
- Peripheral motor neuropathy was observed in 0.6% of patients.
- Grade ≥3 peripheral neuropathy was reported in 1.2% of patients.
- No cases were fatal, and the rate of incidence decreased over time.
Dose adjustment and discontinuation
A total of 36.1% of patients had ≥1 AE that led to dose adjustment; the most common of these are shown in Figure 3.
Figure 3. Most common adverse events that led to dose adjustment*
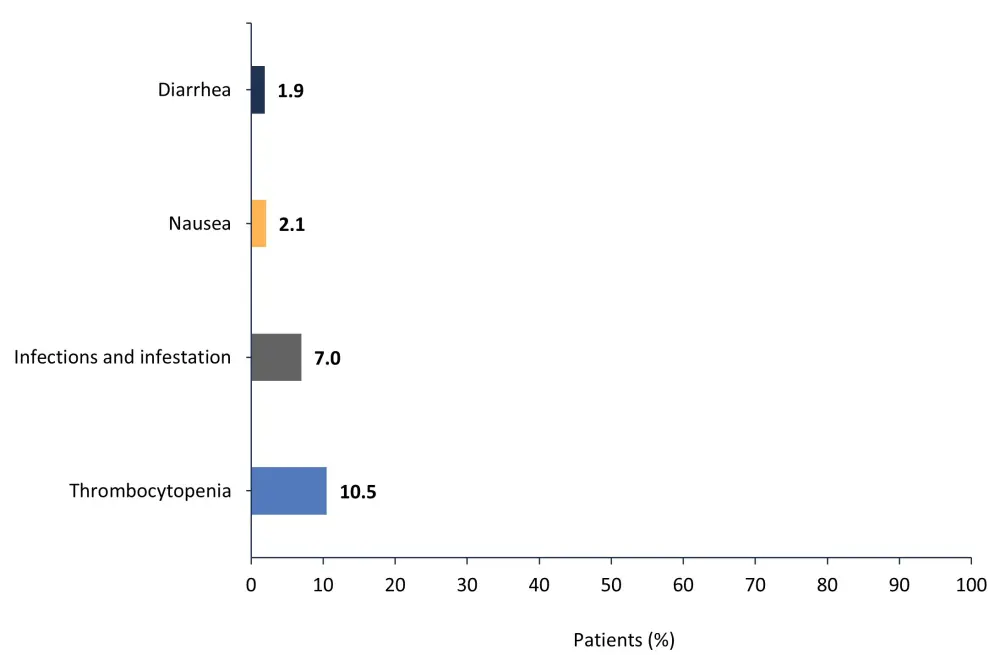
*Adapted from Verstovsek, et al.1
A total of 31.6% of patients had ≥1 AE that led to treatment discontinuation; the most common of these are shown in Figure 4.
Figure 4. Most common adverse events that led to treatment discontinuation*
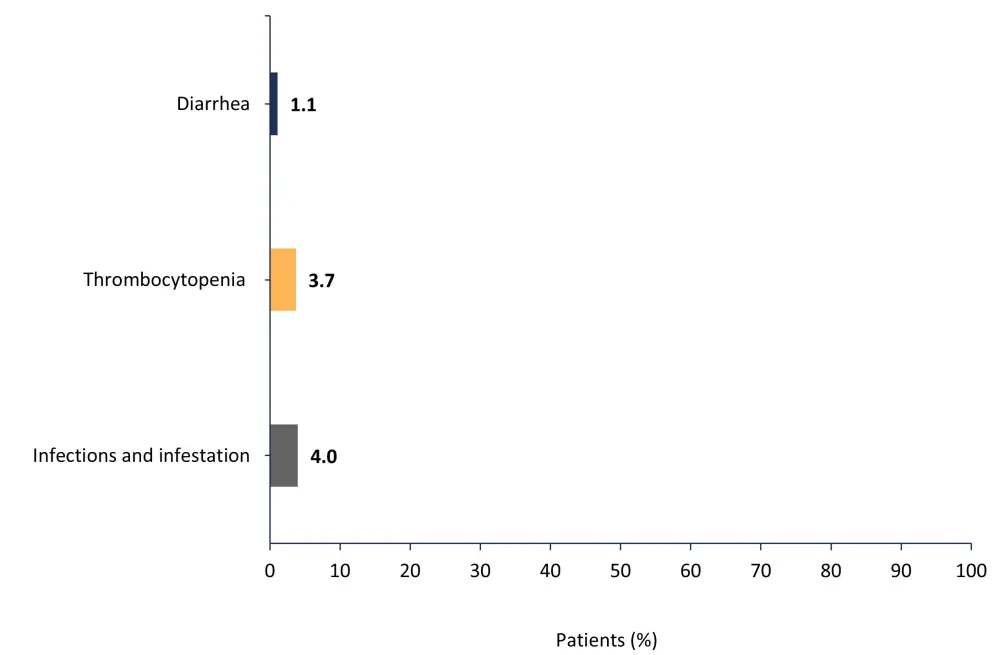
*Adapted from Verstovsek, et al.1
Fatal AEs were reported in 14.1% of patients.
Overall survival and hematologic values
- The median follow-up time for patients across the trials was 3 years.
- The median overall survival (OS) was not reached in the pooled patient population.
- The 2-year OS rate was 76.5%.
- The 4-year OS rate was 59.6%.
- The 6-year OS rate was 51.1%.
- Mean hemoglobin levels increased in each momelotinib-treated patient population and were maintained over time.
- The incidence of anemia did not increase over time.
- Mean neutrophil counts differed between the studies but did not show consistent trends over time when compared with baseline values.
- Neutropenia was uncommon in momelotinib-treated patients and the rate of incidence decreased over time.
Conclusion
Findings from this pooled data analysis show that most AEs were of Grade 1 or 2 severity and did not worsen over time. There was no time trend suggesting late onset or cumulative toxicity with continued treatment. Furthermore, the incidence of most toxicities decreased over time despite continued dose intensity and administration. Overall, the results highlight that continued momelotinib treatment is tolerated in most patients and represents a positive benefit-risk balance in patients diagnosed with intermediate and high-risk MF.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content





