All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Combination therapies in myelofibrosis: Latest considerations on toxicity management with JAK inhibitors
The U.S. Food and Drug Administration (FDA) approval of ruxolitinib monotherapy for intermediate/high-risk myelofibrosis (MF) in 2011 represented a breakthrough in MF treatment.1,2 Approval of two further Janus kinase inhibitor (JAKi) therapies followed, with fedratinib in 2019 and pacritinib in 2022.3
Despite these advances in MF treatment, frontline JAKi monotherapy does not represent long-term disease control or a cure, and may fail due to primary resistance, secondary resistance (relapse), suboptimal response, disease progression, or treatment-related toxicities.2
Therefore, there is an urgent need to introduce novel agents and combination therapies with the potential for disease modification (as indicated by spleen and symptom reduction, bone marrow fibrosis reduction, variant allele fraction reduction, and increased overall survival) to help improve patient outcomes when transplant is not an option.2
In this article, the MPN Hub summarizes three key combination therapies currently being investigated in MF, as presented by Naveen Pemmaraju2 and Aaron Gerds3 at the 1st Congress on Myeloproliferative Neoplasms Controversies and Debates (MPNCo&D). We focus on current considerations on toxicities in combination therapies, and their impact on ongoing clinical development of JAKi-based combinations for the treatment of MF.
Latest developments of JAKi combination therapies for MF2
The overarching objectives of combination therapies in MF are to help minimize toxicities, maximize overall survival, and potentially demonstrate a major benefit over JAKi monotherapy. Table 1 summarizes three key phase III randomized trials on JAKi-based combinations for patients with MF as presented by Pemmaraju at MPNCo&D.
Table 1. Current phase III trials investigating JAKi-based combination therapies in MF*
|
BCL-xLi, B-cell lymphoma-extra large inhibitor; BETi, bromodomain and extraterminal domain inhibitor; JAKi, Janus kinase inhibitor; MF, myelofibrosis; PI3Ki, phosphoinositide 3-kinase inhibitor. |
||
|
Combination |
Phase III trial |
|
|---|---|---|
|
Frontline |
Suboptimal JAKi add-on/add back |
|
|
Ruxolitinib + pelabresib (BETi) |
MANIFEST-2 (NCT04603495) |
|
|
Ruxolitinib + parsaclisib (PI3Ki) |
LIMBER-313 (NCT04551066) |
LIMBER-304 (NCT04551053) |
|
Ruxolitinib + navitoclax (BCL‑xLi) |
TRANSFORM-1 (NCT04472598) |
TRANSFORM-2 (NCT04468984) |
For more details on these ongoing clinical studies and other combination therapies currently being investigated in MF, see our previous review of investigational combination approaches.
JAKi-related toxicities3
To understand the current challenges presented by the use of combination therapies, it is important to consider the key toxicities observed with currently approved JAKi therapies, which form the backbone of such combinations. Hematologic toxicities, such as myelosuppression and cytopenia, are common treatment-related adverse events (Figure 1).
Figure 1. Summary of hematologic toxicities associated with ruxolitinib and pacritinib treatment*
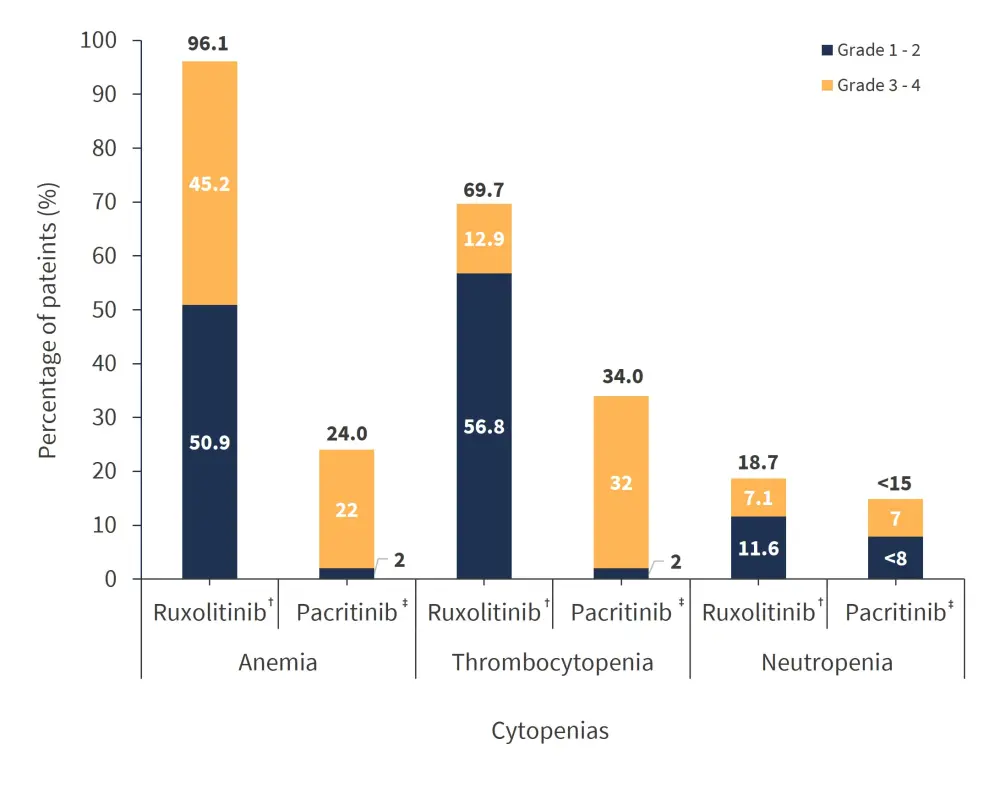
*Data from Gerds.3
†Data from the COMFORT-I trial (N = 155).
‡Data from the PERSIST-2 trial (N = 106).
Other non-hematologic toxicities such as fatigue, bruising, headaches, and gastrointestinal toxicities (diarrhea, vomiting) are also relatively common with JAKi therapy. In addition, agent-specific toxicities have been reported, for example, bleeding and cardiac events with pacritinib (14% and 7% of patients, respectively) and Wernicke's encephalopathy with fedratinib (10% of patients).
An additional JAKi, momelotinib, may be approved in 2023 based on the results of the SIMPLIFY and MOMENTUM trials. Momelotinib may demonstrate reduced anemia, but peripheral neuropathy and other emerging toxicities may present different challenges.
Toxicities in JAKi combination therapies: A case study3
Further considerations are related to cumulative and overlapping toxicities of potential combination agents. Gerds presented a case study of ruxolitinib plus lenalidomide as an example of JAKi-based combination therapies. The combination was potentially attractive considering the expected outcomes of each individual agent (Table 2). However, the cumulative burden of agent-specific toxicities and key overlapping toxicities (Table 2) presented a significant challenge in clinical practice.
Table 2. Clinical outcomes and key toxicities of ruxolitinib and lenalidomide treatment*
|
QoL, quality of life. |
||
|
Treatment |
Treatment-specific clinical outcomes |
Key overlapping toxicities |
|---|---|---|
|
Ruxolitinib |
Reduces spleen size |
Cytopenia |
|
Lenalidomide |
30% response rates |
|
The ruxolitinib plus lenalidomide combination improved response rates as compared with JAKi monotherapy, but the majority of patients (87%) required lenalidomide discontinuation due to severe adverse events, with only 30% being able to restart lenalidomide treatment. A total of 45% of patients discontinued lenalidomide within the first 3 months of treatment, with myelosuppression being a key element of limited treatment-related tolerability.
This example illustrates how combination therapy with JAKi may present a potential attractive opportunity to help improve clinical outcomes; however, cumulative toxicities can be too great for a viable treatment.
Conclusion
JAKi combination therapies represent a potential attractive therapeutic option to help improve patient outcomes in MF and overcome certain limitations of monotherapies. However, cumulative treatment toxicities, and especially overlapping toxicities currently represent a challenge to developing viable combination regimens in clinical practice. Limited tolerability profiles are identified as a key limiting factor for JAKi combination therapies and may present an opportunity for future therapeutic advancements. Future developments of successful JAKi-based combination therapies for patients with MF will need to consider non-overlapping toxicity profiles within the context of clinical studies and specific patient populations in which these emerging therapies are investigated.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




