All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
SIMPLIFY-1: Update on momelotinib for myelofibrosis
Your opinion matters
Dysregulation in JAK-STAT and ACVR1 signaling contributes to disease manifestations of myelofibrosis.
Momelotinib, a potent Janus kinase 1 and 2 (JAK1/2) inhibitor (JAKi) and an inhibitor of activin A receptor type I (ACVR1; also known as ALK-2), is being evaluated for the treatment of myelofibrosis (MF). The SIMPLIFY-1 trial (NCT01969838) compared momelotinib vs ruxolitinib in JAKi-naïve patients with MF where patients were randomized to receive momelotinib or ruxolitinib for 24 weeks, and open label momelotinib was available for all patients after Week 24.1
In an update of the SIMPLIFY-1 trial, which was recently published by Mesa et al.1 in the Leukemia & Lymphoma journal, retrospective, dynamic, and time-to-event analyses were performed on JAKi-naïve patients with MF. We summarize updated results below.
The MPN Hub previously provided an update of the SIMPLIFY-1 and SIMPLIFY-2 trials (best available therapy or ruxolitinib were used for comparison; NCT02101268). Momelotinib produced greater rates of transfusion independence, splenic response, and a reduction of constitutional symptoms in patients with myelofibrosis (MF) compared with ruxolitinib, which were associated with greater overall survival (OS).2,3
Results
Hemoglobin concentrations
- Treatment with momelotinib produced a rapid increase in mean hemoglobin (<1 g/dL) that was significantly greater by Week 24 compared to treatment with ruxolitinib (p < 0.001).
- The mean hemoglobin levels significantly decreased from baseline to Week 24 in the ruxolitinib cohort (p < 0.001).
- When patients receiving ruxolitinib were switched to momelotinib at Week 24, median hemoglobin levels rapidly increased and were comparable to those from patients who received momelotinib from Week 1.
Transfusion independence
- A zero-inflated negative binomial model was used to compare the proportion of patients with zero transfusions throughout the randomized treatment period.
- The probability of zero transfusions based on various baseline covariates identified is summarized in Table 1.
- Notably, the odds of achieving zero transfusions were 9.69 times higher when receiving momelotinib versus ruxolitinib.
- The probability for achieving zero transfusion units was consistently higher in baseline covariates.
Table 1. Probability of zero transfusions (least squares mean) for the momelotinib and ruxolitinib cohorts*
|
BMF, bone marrow fibrosis; INT, international prognostic scoring system; MF, myelofibrosis; PET-MF, post-essential thrombocythemia myelofibrosis; PMF, primary myelofibrosis; PPV-MF; post-polycythemia vera myelofibrosis; RBC, red blood cell. |
||
|
Variable |
Momelotinib |
Ruxolitinib |
|---|---|---|
|
Overall |
0.83 |
0.34 |
|
Baseline BMF |
||
|
Grade 1 |
0.96 |
0.73 |
|
Grade 2 |
0.88 |
0.43 |
|
Grade 3 |
0.76 |
0.25 |
|
Baseline INT |
||
|
1 |
0.97 |
0.79 |
|
2 |
0.89 |
0.45 |
|
Baseline high |
0.60 |
0.13 |
|
PET-MF |
0.79 |
0.28 |
|
PPV-MF |
0.90 |
0.48 |
|
PMF |
0.82 |
0.32 |
|
RBC units |
||
|
No baseline |
0.94 |
0.61 |
|
1–3 baseline |
0.32 |
0.05 |
|
4+ baseline |
0.06 |
0.01 |
- The benefit of momelotinib for achieving zero transfusion units was also demonstrated using a Kaplan-Meier (KM) analysis of the time-to-first, time-to-third, and time-to-fifth red blood cell (RBC) units (Figure 1).
- The analysis also demonstrated that patients randomized to momelotinib were 3.6 times more likely to require <3 transfusion units by Week 24 versus those receiving ruxolitinib.
- The odds of receiving <5 transfusion units by Week 24 were 3.0 times greater in the momelotinib group compared with ruxolitinib.
Figure 1. Probability of achieving zero, <3, and <5 transfusion units by Week 24*
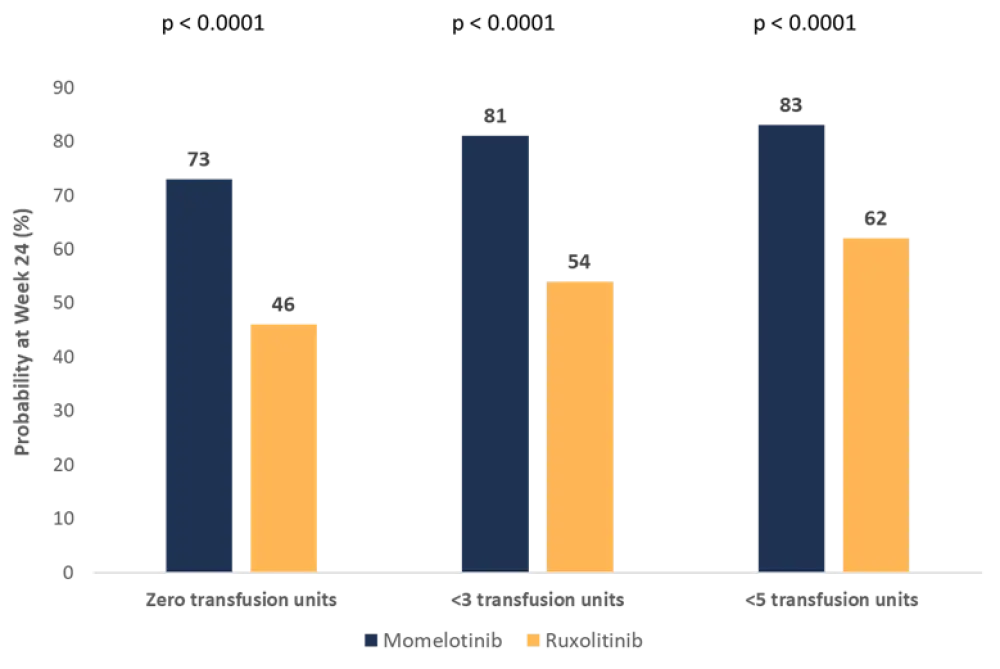
*Adapted from Mesa et al.1
- Notably, the time-to-loss of transfusion dependence was not reached in patients receiving momelotinib, indicating that this treatment was durable.
- The investigators performed a cumulative transfusion burden analysis using recurrent events that revealed that patients receiving ruxolitinib received twice as many RBC transfusions at any time point compared with those on momelotinib (HR, 0.522; p < 0.0001)
Conclusions
The updated analysis of the randomized and open label treatment period from SIMPLIFY-1, further clarified the benefit of momelotinib for transfusion requirements in patients with MF when compared with ruxolitinib. Momelotinib is further being investigated in the phase III MOMENTUM trial (NCT04173494), which recruited symptomatic and anemic patients previously treated with an approved JAKi. Earlier this year, it was announced that the MOMENTUM trial met its primary and major secondary endpoints; read more here.
Additional content
Click here to watch a video interview with Ruben A. Mesa, entitled ‘SIMPLIFY-1+2 trial: What is the long-term benefit of momelotinib treatment in R/R MF?’
Click here to watch a video interview with Ruben A. Mesa, entitled ‘How does transfusion independence impact survival outcomes with momelotinib in MF?’
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

