All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Overall survival with imetelstat versus best available therapy with real-world data in patients with R/R MF
Myelofibrosis (MF) is a heterogeneous disease where outcomes vary among patients. As such, the Dynamic International Prognostic Scoring System (DIPSS) score of intermediate-2 or high-risk MF is associated with poorer median overall survival (OS) compared with low- and intermediate-1 risk disease, and accounts for almost 60% of patients with MF. In the treatment landscape, JAK inhibition plays a critical role in symptom improvement and spleen volume reduction; however, it may not be the most appropriate option for all patients with MF.
Patients with MF who are relapsed/refractory (R/R) to JAK inhibitors (JAKi) have poor outcomes and no standard treatment approach. Imetelstat, a first-in-class, potent inhibitor of telomerase enzymatic activity, is currently under investigation for this patient population with advanced disease. The MPN Hub Steering Committee member John Mascarenhas and colleagues reported the latest results of MYF2001 (IMbark; NCT02426086), a randomized phase II trial of imetelstat in patients with MF who are R/R to JAKi. This trial demonstrated encouraging results with improved clinical benefit and OS in patients receiving 9.4 mg/kg every 3 weeks of imetelstat compared with those receiving 4.7 mg/kg of imetelstat.1
Here we summarize the findings of a recent study by Kuykendall et al.2 published in Annals of Hematology, further exploring the potential OS benefit of 9.4 mg/kg of imetelstat in patients enrolled in the MYF2001 trial compared with a closely matched cohort from real-world data (RWD) treated with best available therapy (BAT).
Study design
This was an observational, retrospective cohort study with a closely matched control-cohort. Patients in the study cohort were those who had participated in MYF2001 trial based on the eligibility criteria:
- worsening of splenomegaly-related abdominal pain at any time after start of JAKi AND no improvement in spleen size or volume following 12-week JAK inhibition OR worsening of splenomegaly at any time after the initiation of JAK inhibition
- active symptoms of MF
- baseline measurable splenomegaly (palpable spleen ≥ 5 cm below left coastal margin or ≥ 450 cm3 by magnetic resonance imaging)
The matched historical control-cohort was identified using the MYF2001 eligibility criteria and comprised of RWD for 96 patients with MF who had discontinued ruxolitinib (exclusion of discontinuation due to intolerance or toxicity), who had persistent or worsening splenomegaly despite ruxolitinib therapy, and subsequently treated with BAT at the Moffitt Cancer Center between 2010–2018. The most common BAT after ruxolitinib was the combination of lenalidomide and prednisone.
The study population (study- and control-cohort) was balanced in terms of important baseline covariates and prognostic factors that may impact OS (age, platelet count, time from diagnosis to JAKi discontinuation, JAKi treatment duration, spleen size, JAK2 mutation status, sex, DIPSS score, Eastern Cooperative Oncology Group [ECOG] performance status, MF subtype, and red blood cell transfusion status), using a propensity score analysis approach, which was achieved with:
- average treatment effect for overlap (ATO) population
- stabilized inverse probability treatment weighting (sIPTW)
The primary outcome of interest was OS measured from the time of JAKi discontinuation to death or censored at last follow-up.
Results
Baseline characteristics
The study population comprised of 57 patients treated with imetelstat 9.4 mg/kg from MYF2001 and 38 patients treated with BAT from RWD (Figure 1).
Figure 1. Study population*
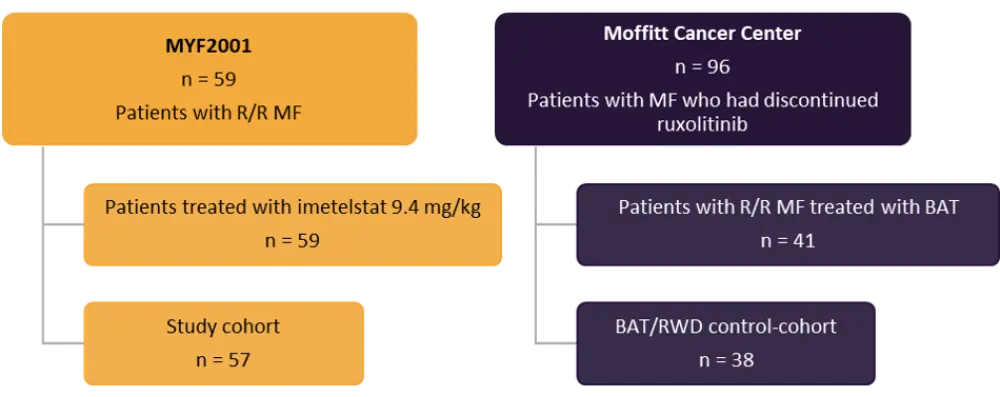
BAT, best available therapy; MF, myelofibrosis; R/R, relapsed/refractory; RWD, real-world data.
*Adapted from Kuykendall et al.2
- The baseline covariates including spleen size, transfusion dependence, time from diagnosis to JAKi discontinuation, JAKi treatment duration, and JAK2 mutation status (Table 1) showed statistically significant differences between the two groups without adjustment.
- However, after adjustment for baseline covariate these differences were balanced using the ATO method (standardized mean differences were < 0.001 for all) as well as the sIPTW method (standardized mean differences were < 0.3 for all).
Table 1. Baseline characteristics before and after adjustment*
|
ATO, average treatment effect for overlap population; BAT, best available therapy; DC, discontinuation; DIPSS, Dynamic International Prognostic Scoring System; ECOG, Eastern Cooperative Oncology Group; ET, essential thrombocythemia; JAK, Janus kinase; JAKi, Janus kinase inhibitor; MF, myelofibrosis; PV, polycythemia vera; RWD, real-world data; SD, standard deviation. |
||||||
|
Characteristic, % (unless otherwise stated) |
Before adjustment† |
After adjustment |
||||
|---|---|---|---|---|---|---|
|
Imetelstat 9.4 mg/kg |
BAT/RWD |
p value |
Imetelstat 9.4 mg/kg |
BAT/RWD |
p value |
|
|
Mean age, years, (SD) |
66.4 |
67.8 |
0.488 |
66.6 |
66.6 |
1.000 |
|
Sex, female/male |
40/60 |
34/66 |
0.127 |
39/61 |
39/61 |
1.000 |
|
MF subtype |
||||||
|
Primary MF |
60 |
68 |
0.184 |
69 |
69 |
1.000 |
|
Post-ET/post-PV |
40 |
32 |
|
31 |
31 |
|
|
DIPSS risk status |
||||||
|
Intermediate |
58 |
71 |
0.278 |
64 |
64 |
1.000 |
|
High |
42 |
29 |
|
36 |
36 |
|
|
Mean spleen size (SD) |
17.2 |
13.1 |
0.009 |
15.0 |
15.0 |
1.000 |
|
Mean platelet count (× 109/L) (SD) |
212.1 |
169.5 |
0.154 |
178.5 |
178.5 |
1.000 |
|
ECOG |
||||||
|
0–1 |
81 |
63 |
0.096 |
73 |
73 |
1.000 |
|
2–3 |
19 |
37 |
|
27 |
27 |
|
|
Transfusion dependent |
21 |
42 |
|
32 |
32 |
|
|
Mean time from diagnosis to JAKI DC, months (SD) |
55.2 |
26.9 |
0.001 |
34.7 |
34.7 |
1.000 |
|
Mean duration of JAKI treatment, months (SD) |
25.1 |
13.9 |
0.002 |
16.8 |
16.8 |
1.000 |
|
JAK2 V617F |
||||||
|
Negative |
44 |
18 |
0.019 |
26 |
26 |
1.000 |
|
Positive |
56 |
82 |
|
74 |
74 |
|
Overall survival
- The median follow-up was 23 vs 43 months in patients treated with imetelstat 9.4 mg/kg and BAT, respectively.
- Imetelstat showed statistically significant reductions in death across all analyses compared with BAT (hazard ratio 0.35; p = 0.0019)
- The median OS was 33.8 vs 12.0 months in the unweighted analysis and 30.7 vs 12.0 months in the overlap weighting analysis with ATO in the imetelstat and BAT group, respectively.
- The median OS with imetelstat was consistent using sIPTW with ATO method and the corresponding reduction in the risk of death was 67%.
- Sensitivity analyses demonstrated 65%, 64% and 66% lower risk of death with imetelstat vs BAT in the unweighted analysis, and ATO and sIPTW, respectively, an observation consistent with primary findings.
- The impact of autologous hematopoietic cell transplantation (auto-HCT) after imetelstat or BAT evaluated by censoring at the transplant and showed an improved median OS of 30.7 vs 10.2 months with imetelstat compared to BAT and a 69% reduction in the risk of death in the unweighted analyses.
- Similarly, the reduction in death with imetelstat was 68% and 70% respectively, using ATO and sIPTW.
Conclusion
This study demonstrated improved median OS exceeding 30 months along with lower risk of death with imetelstat compared with BAT using RWD of patients with intermediate-2 or high-risk MF after treatment failure with ruxolitinib. The primary and sensitivity analyses consistently showed improved OS with imetelstat compared with BAT. The propensity score approach allowed the separation of an observational study from its analysis, estimated the marginal treatment effect, and examined the degree of overlap in the distribution of baseline covariates in both groups. However, the study was also limited by confounding bias, small sample size, and the challenge of comparing outcomes from clinical study patients with real-world patients. A prospective evaluation is therefore warranted, and a phase III randomized trial evaluating OS in this poor-prognosis patient population is currently underway (NCT04576156).
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

