All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Do you know... Which of the following JAK inhibitor(s) has demonstrated efficacy in patients with myelofibrosis and platelet count <50,000/µL?
During the European School of Haematalogy (ESH) 4th How to Diagnose and Treat CML/MPN conference, the MPN Hub held a symposium on March 9, 2025, titled, Anemia in myelofibrosis: Sequencing therapies to optimize patient outcomes. Here, we share a presentation by Paola Guglielmelli, University of Florence, Florence, IT, discussing the optimal sequencing of therapies in patients with myelofibrosis and anemia..
Sequencing therapies: Optimizing treatment for myelofibrosis
Sequencing therapies: Optimizing treatment for myelofibrosis
Guglielmelli discussed the differences between myeloproliferative and cytopenic myelofibrosis (MF), highlighting the limited treatment options in patients with severe cytopenias.1,2 She discussed the British Society of Haematology and National Comprehensive Cancer Network guidelines for the treatment of anemia in patients with MF, which both suggest the use of momelotinib in patients with MF and anemia and symptomatic splenomegaly.3,4
Guglielmelli then presented three patient cases and discusses the clinical evidence influencing treatment decisions specific to each case (Figures 1–3).
Patient case 1
Figure 1. Patient case 1*

- A real-world study demonstrated that ruxolitinib-treated patients with cytopenia MF (n = 479) have worse survival outcomes vs patients with myeloproliferative MF (n = 407; median overall survival, 4.1 years vs 7.6 years; p < 0.001).6
- A retrospective analysis of the phase III SIMPLIFY-1 trial (NCT01969838) showed that 60% of patients treated with ruxolitinib required a dose modification, and at the time of crossover, the majority of patients who switched to momelotinib did not require tapering or a washout and started momelotinib at the optimal 200 mg dose.7
- Hemoglobin (Hb) levels rapidly recovered, and transfusion independence (TI) rates increased, while spleen response was maintained in patients who transitioned from ruxolitinib to momelotinib.7
Patient case 2
Figure 2. Patient case 2*
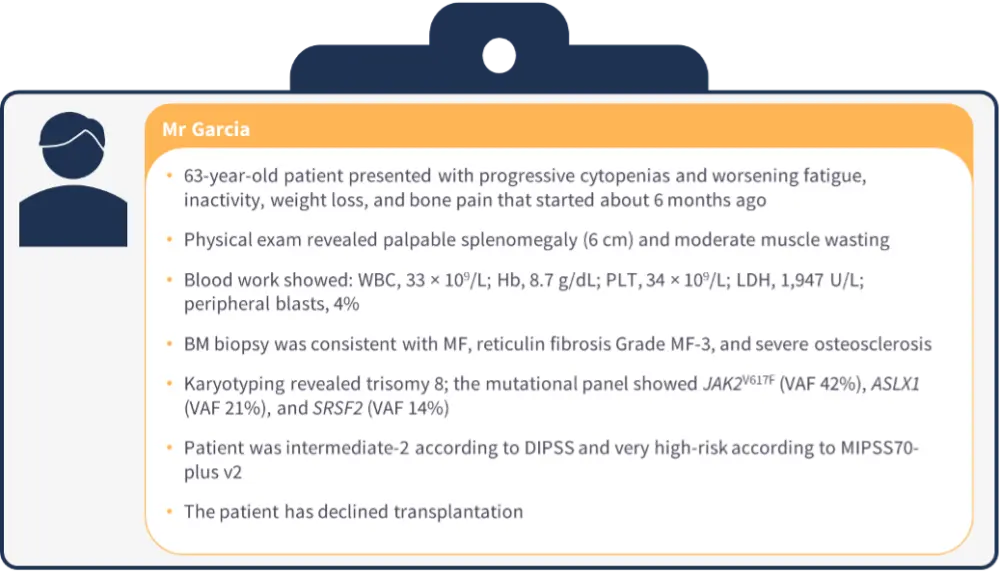
- Ruxolitinib and fedratinib are contraindicated for patients with a platelet (PLT) count <50 × 109/L.
- A pooled analysis of the phase III PERSIST-1 (NCT01773187) and PERSIST-2 (NCT02055781) trials demonstrated the benefit of pacritinib in patients with severe thrombocytopenia.9
- An analysis of patients with a PLT count <50 × 109/L in the phase III MOMENTUM trial (NCT04173494) showed that momelotinib is effective in this patient population, suggesting that momelotinib is an alternative for patients with lower PLT counts (≥25 × 109/L).10
Patient case 3
Figure 3. Patient case 3*
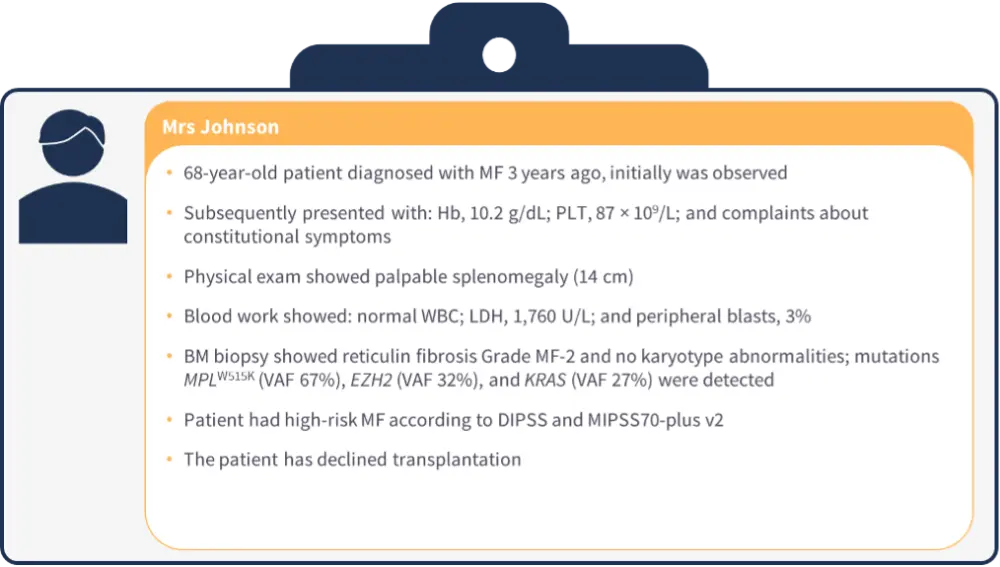
- Results from subanalyses of the SIMPLIFY-1 trial showed that in patients with mild and moderate anemia, spleen and symptom response rates were similar between momelotinib and ruxolitinib, while TI rates were improved with momelotinib (Hb <10 g/dL, 47% vs 27%; Hb 10–12 g/dL, 81% vs 51%).11
- Among patients with PLTs 50–100 × 109/L, patients randomized to momelotinib had improved 35% spleen volume reduction rates (39% vs 0) and TI rates (61% vs 39%) vs ruxolitinib.10
Conclusion
Guglielmelli concluded by presenting a treatment algorithm for the selection of Janus kinase inhibitors for patients with MF based on cytopenia (Figure 4).
Figure 4. Selection of JAK inhibitors in patients with MF*
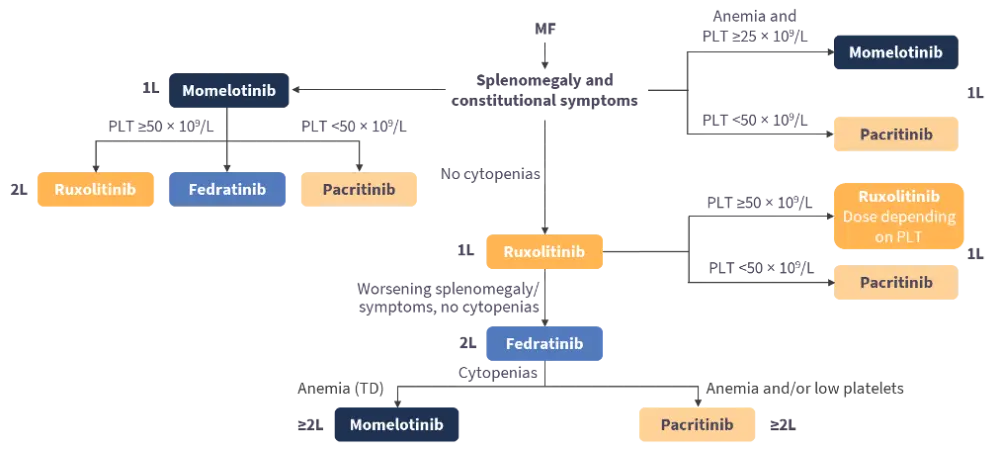
This independent educational activity is supported by GSK. All content is developed independently by the faculty; the funder is allowed no influence on the content.
Your opinion matters
What do you consider to be the highest unmet need for patients with myelofibrosis?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Paola Guglielmelli
Paola Guglielmelli


