All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
TRANSFORM-1: Key safety and efficacy results
Do you know... The primary endpoint of the TRANSFORM-1 study was spleen volume reduction ≥35% (SVR35) at Week 24. What percentage of patients treated with navitoclax + ruxolitinib achieved the primary endpoint?
Ruxolitinib (RUX) monotherapy is currently the standard of care for patients diagnosed with myelofibrosis, with spleen volume reduction ≥35% (SVR35) at Week 24 consistently in the range of 29%–49%.1 While patients experience symptom improvement and spleen volume reduction ≥35%, limited benefit remains in key clinical outcomes.1 Navitoclax (NAV), a novel oral B-cell lymphoma-extra-large and B-cell lymphoma-2 inhibitor in combination with RUX has shown antitumor activity and clinical benefit in the recent phase II REFINE trial (NCT03222609).
At the 65th American Society of Hematology (ASH) Annual Meeting and Exposition, Pemmaraju presented key efficacy and safety data from the phase III TRANSFORM-1 study (NCT04472598), the first randomized clinical trial investigating NAV + RUX combination compared with RUX + placebo (PBO) in patients with Janus kinase inhibitor-naïve myelofibrosis. We summarize the key points below.
Study design1
- A total of 252 patients were enrolled in the study and randomized 1:1
- NAV 100/200 mg once daily + RUX 15/20 mg twice daily (n = 125)
- RUX 15/20 mg twice daily + PBO (n = 127)
- The primary endpoint was SVR35 at Week 24
- Secondary endpoints included SVR35 at any time, duration of SVR35, and change in Total Symptom Score (TSS) from baseline to Week 24
- Patient eligibility criteria are shown in Table 1
Table 1. Eligibility criteria*
|
ECOG, European Co-operative Oncology Group; JAK, Janus kinase; MF, myelofibrosis. |
|
Eligibility criteria |
|---|
|
Age ≥18 years |
|
ECOG performance score ≤ 2 |
|
Intermediate-2 or high-risk MF |
|
Measurable splenomegaly |
|
Evidence of symptoms |
|
No prior JAK inhibitor therapy |
Results1
- Baseline patient characteristics were similar between the two treatment arms (Table 2)
- The median follow-up was 14.9 months
Table 2. Baseline patient characteristics*
|
CALR, calreticulin; DIPSS+, Dynamic International Prognostic Scoring System plus; HMR, high molecular risk; JAK, Janus kinase; MPL, ; myeloproliferative leukemia virus oncogene; NAV, navitoclax; PBO, placebo; RUX, ruxolitinib; TSS, Total Symptom Score. |
||
|
Characteristic, % (unless otherwise stated) |
NAV + RUX (n = 125) |
RUX + PBO (n = 127) |
|---|---|---|
|
Age, years |
70 |
69 |
|
Sex, male |
50 |
64 |
|
Number of prior lines of therapy, median (range) |
1 |
1 |
|
Median spleen volume, cm3 |
1,441 |
1,639 |
|
Median TSS |
21 |
24 |
|
DIPSS+ risk |
||
|
Intermediate-1 |
6 |
4 |
|
Intermediate-2 |
83 |
87 |
|
High-risk |
10 |
9 |
|
Driver mutation |
||
|
JAK2V617F |
65 |
62 |
|
CALR |
18 |
20 |
|
MPLW515 |
11 |
8 |
|
HMR mutations |
48 |
43 |
Efficacy
- The median time to first SVR35 response was similar in both treatment groups
- 12.3 months in the combination group vs 12.4 months in the placebo group
- SVR35 at Week 24 was twice as high in the combination group vs the placebo group (Figure 1)
Figure 1. SVR35 response at Week 24 and at any time*
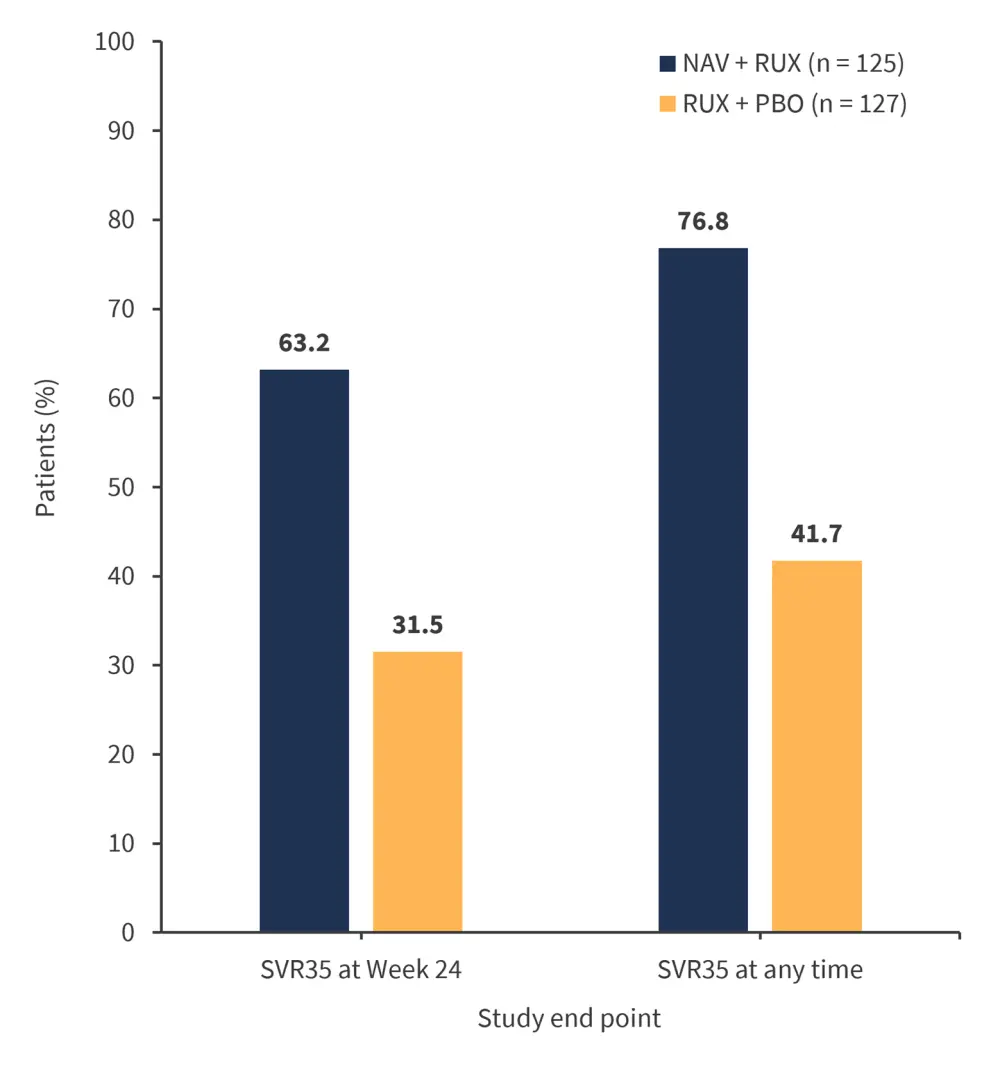
NAV, navitoclax; PBO, placebo; RUX, ruxolitinib; SVR35, spleen volume reduction ≥35%.
*Adapted from Pemmaraju.1
- The mean change in TSS from baseline at Week 24 in the combination group was 9.7 vs 11.1 in the placebo group (p = 0.2852).
- Reduction in TSS ≥50% at Week 24 was experienced by 39.2% of patients in the combination group vs 41.7% of patients in the placebo group.
- NAV + RUX doses were higher in responders vs non-responders
Safety
- 33% of patients discontinued treatment
- The most common cause of discontinuation was adverse events (AEs), 14% in the NAV + RUX group and 11% in the RUX + PBO group
- The most common AEs of any grade experienced by >30% of patients receiving NAV are shown in Figure 2
Figure 2. Most common AEs of any grade experienced by 30% of patients receiving NAV*
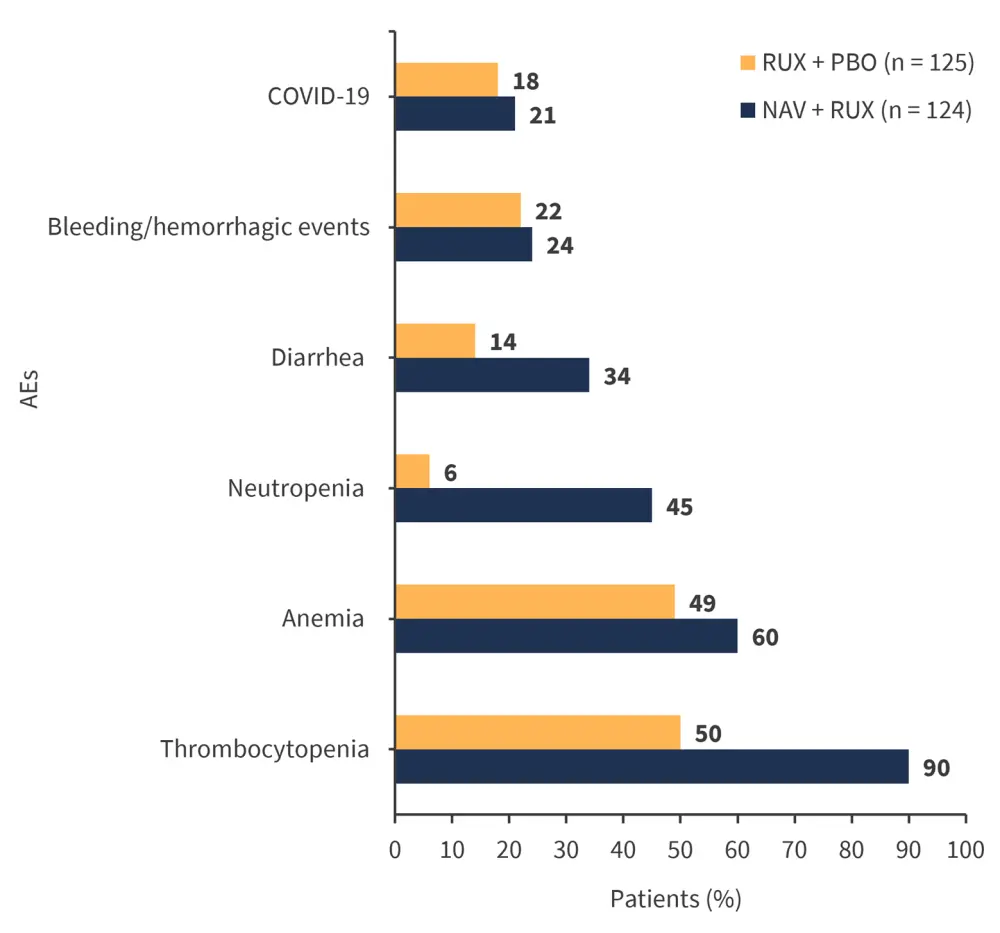
AE, adverse event; NAV, navitoclax; PBO, placebo; RUX, ruxolitinib.
*Adapted from Pemmaraju.1
- Grade ≥3 AEs were experienced by 85% of patients in the combination group and 70% of patients in the placebo group
- Serious AEs were experienced by 26% of patients in the combination group and 32% of patients in the placebo group
- Dose interruptions or reductions were mainly due to thrombocytopenia
- None were due to bleeding events
Conclusion
This was the first randomized clinical trial investigating NAV + RUX in this setting. SVR35 at Week 24 was doubled for patients treated with combination therapy compared with RUX + PBO. AEs were manageable, mainly through dose modification. Further evaluations are ongoing to investigate overall survival and clinical responses in patient subgroups.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


