All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Myelofibrosis: Current combination therapies for JAKi-naïve patients
Do you know... Ruxolitinib is an essential treatment for patients diagnosed with MF; however, there remains a risk of relapsed or refractory disease. What percentage of patients discontinue ruxolitinib therapy after 3 years?
Janus kinase inhibitor (JAKi) therapy has been the cornerstone of myelofibrosis (MF) treatment for the past decade. Identification of the key driver mutation in the JAK2 gene has facilitated a continuous improvement in survival rates and symptom responses for patients diagnosed with MF.
However, there remain challenges in optimizing response rates and managing the side effects of JAKi treatment; this has led to the investigation of combination therapies, as previously covered by the MPN Hub. The aim is to enhance the effects of initial JAKi treatment with a combined novel treatment, minimizing new toxicity signals and improving overall survival (OS) for patients with MF.
During the 2nd Myeloproliferative Neoplasms (MPN) Controversies and Debates, Pemmaraju presented current data on combination therapies in JAKi-naïve patients diagnosed with MF, highlighting recent results from clinical trials investigating combination treatments and outlining future directions in the field. Here, we summarize the key points.
Why do we need JAKi therapy combinations?1
- Initially, the COMFORT-1 phase II study (NCT00934544), investigating ruxolitinib (RUX) versus placebo, represented a breakthrough in MF treatment.
- The primary endpoint of spleen volume reduction ≥35% (SVR35) at Week 24 was met in 41.9% and 0.7% of patients treated with RUX and placebo, respectively (p < 0.001)
- Symptom response at Week 24 was experienced by 45.9% of patients treated with RUX versus 5.3% of patients treated with placebo (p < 0.001).
- However, around 41% of patients discontinue RUX therapy after 3 years due to several factors, such as refractoriness and suboptimal response (Figure 1).
Figure 1. Reasons for RUX discontinuation*
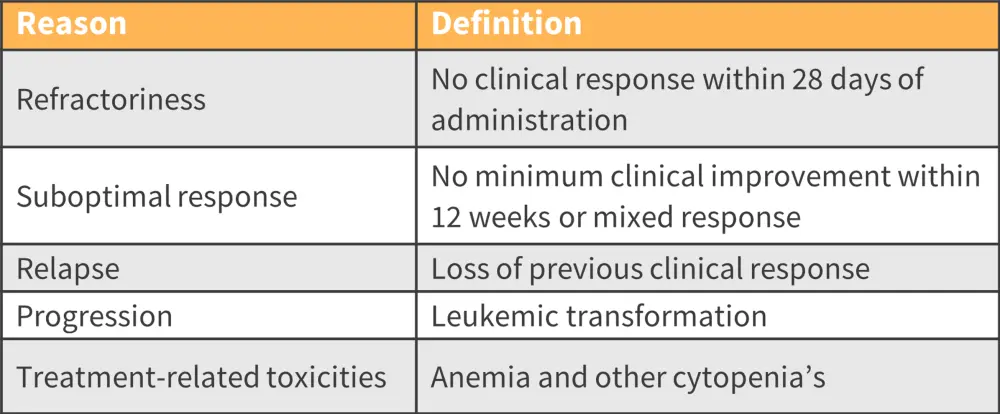
RUX, ruxolitinib.
*Adapted from Pemmaraju.1
The discontinuation rate highlights the need for therapeutic options beyond JAKis, such as the use of combination treatments and targeting new pathways, including:
- promotion of apoptosis;
- targeting of the hematopoietic stem cell micro-environment;
- activation of the TP53 pathway;
- targeting fibrosis, cytokines, epigenetics, and other pathways;
- telomerase inhibition; and
- targeting and improving anemia in MF.
New combination therapies1
Pelabresib + RUX
- In Arm 3 of the phase III MANIFEST trial (NCT04603495) investigating RUX + pelabresib, SVR35 at Week 24 was 68%,
- SVR35 at any timepoint was 80%;
- ≥50 percent reduction in total symptom score at Week 24 was 56%; and
- ≥50 percent reduction in total symptom score at any timepoint was 83%.
- Evidence of disease modification was seen in 27% of patients experiencing ≥1 grade improvement in bone marrow fibrosis (BMF) at Week 24.
- 40% of patients experienced ≥1 grade improvement in BMF at any timepoint.
Navitoclax + RUX
- In the phase II REFINE trial (NCT03222609), median OS was not reached after 24 months;
- spleen response was independent of high-risk molecular mutations; and
- OS benefit was observed in patients with improvement in BMF or variant allele frequency reduction—a potential early signal of disease modification.
- Preliminary evidence from the REFINE trial, investigating the combination of navitoclax in JAKi-naïve patients, also demonstrated promising safety and efficacy data:
- All evaluable patients achieved spleen volume reduction and a manageable safety profile.
- A third of patients with SVR35 also experienced a reduction in BMF, with 28% of patients experiencing ≥1 grade improvement at any time and 22% of patients experiencing complete BMF resolution.
Pegylated interferon alfa-2a + RUX
- Combined results from phase I and II of the RUXOPEG trial (NCT02742324) revealed that 70% of patients experienced a 50% spleen volume reduction after 24 weeks; this increased to 76% of patients after 12 months.
- Currently ongoing global phase III studies investigating RUX combination therapies are shown in Table 1.
Table 1. Ongoing global phase III studies investigating RUX combination therapies*
|
BCLXL, B-cell lymphoma-extra-large; BET, bromodomain and extra terminal domain; PI3K, phosphoinositide 3-kinase; RUX, ruxolitinib. |
|||
|
Combination |
Target |
Trial |
NCT |
|---|---|---|---|
|
RUX + pelabresib |
BET inhibitor |
MANIFEST II |
|
|
RUX + navitoclax |
BCLXL inhibitor |
REFINE |
|
|
RUX + parsaclisib |
PI3K inhibitor |
LIMBER-304 |
|
Conclusion
The addition of RUX to novel therapies has the potential to significantly impact the MF treatment landscape, enhancing treatment effects and improving clinical outcomes. Moreover, preliminary evidence suggests that some combination therapies such as RUX + navitoclax and RUX + pelabresib may lead to disease modification through BMF and variant allele reduction.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

