All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Novel agents to treat MF with suboptimal response to ruxolitinib: Combining ruxolitinib with navitoclax or KRT-232
Featured:
Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway dysregulation is central to the pathogenesis of myelofibrosis (MF), giving rise to progressive anemia, splenomegaly, and other, constitutional symptoms in patients with MF.1 The JAK1/JAK2 inhibitor (JAKi), ruxolitinib, was approved for the treatment of patients with primary and secondary intermediate-/high-risk MF in 2011, based on results showing significantly reduced spleen volume, improved MF-related symptoms and quality of life, and prolonged overall survival (OS).2 However, there remains an unmet clinical need for the effective management of patients with MF who do not respond to, or have suboptimal responses to, ruxolitinib and those patients who need to discontinue JAKi therapy due to toxicity.
During the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, there were a number of presentations on the novel agents and treatment combinations under evaluation for patients who have not achieved adequate responses to ruxolitinib. The MPN Hub is happy to present a summary of two abstracts investigating the combination of ruxolitinib with either navitoclax or KRT-232.
Ruxolitinib + navitoclax3
Navitoclax is an orally available inhibitor of the BCL-2 family members, BCL-XL and BCL-2. The novel agent has shown to augment the anti-tumor effect of JAK2 inhibition in preclinical studies and could address ruxolitinib resistance in patients with MF.
Patients with MF who harbor high-molecular-risk (HMR) mutations at the time of diagnosis, such as in ASXL1, SRSF2, EZH2, U2AF1, and IDH1/2, have poor prognosis. An ongoing phase II study (NCT03222609) is evaluating navitoclax either alone or in combination with ruxolitinib for patients with MF whose response to ruxolitinib has diminished. Naveen Pemmaraju, MD Anderson Cancer Center, discussed a sub analysis of this study, which sought to investigate whether:
- HMR mutations, or total number of mutations, are coupled with clinical outcomes in patients treated with navitoclax + ruxolitinib.
- Navitoclax + ruxolitinib can regulate cytokine release and identify the relationship between cytokine levels and clinical outcomes.
Study design
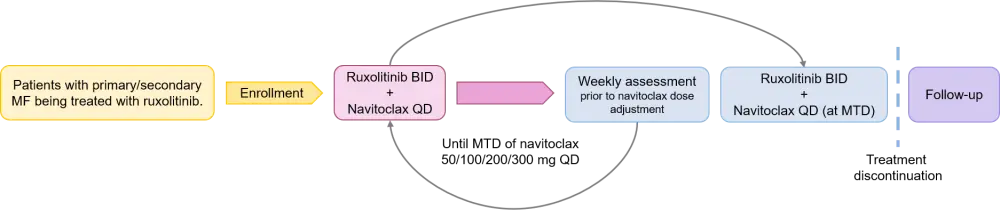
- Single-arm, multicenter, open label, phase II study (Figure 1).
- Patients were eligible if they had primary or secondary MF with splenomegaly and were receiving continuous ruxolitinib but experienced treatment failure at ≥ 12 weeks.
- HMR mutations were defined as mutations in ASXL1, SRSF2, EZH2, U2AF1, and IDH1/2.
- Study endpoints/clinical outcomes were defined as:
- Spleen volume reduction (SVR) at Week 24 (%)
- Total symptom score (TSS) change from baseline to Week 24 (%)
- Change in degree of bone marrow fibrosis from baseline
- Safety
- Prognostic biomarkers
- Mutational analyses and variant allele frequency measurement occurred at baseline and Week 24, and cytokine levels were measured in plasma samples at baseline, Week 12, and Week 24.
Figure 1. Treatment schema for navitoclax + ruxolitinib in patients with MF3
Results
- 34 patients were enrolled with a median age of 68 years (range, 42–86). Patients were mostly male (68%) and had Eastern Cooperative Oncology Group (ECOG) performance status of 0 (47%) or 1 (53%). Baseline samples for biomarker analysis were available for 33 patients. Further patient characteristics are shown in Table 1.
Table 1. Characteristics of patients receiving navitoclax + ruxolitinib3
|
HMR, high mutational risk. |
|
|
Characteristic |
Navitoclax + ruxolitinib (N = 34) |
|---|---|
|
Median duration of prior ruxolitinib treatment, months (range) |
20 (4–97) |
|
Median spleen volume, cm3 (range) |
1,695 (465–5,047) |
|
Mutational status, % |
|
|
JAK2 |
79 |
|
CALR |
21 |
|
HMR mutations, % |
56 |
|
ASXL1 |
68* |
|
SRSF2 |
37* |
|
EZH2 |
21* |
|
U2AF1 |
10* |
|
IDH1 |
5* |
|
≥ 2 HMR mutations, % |
42* |
Efficacy
- At Week 24:
- 27% of patients achieved an SVR of ≥ 35% (SVR35)
- 30% of evaluable patients attained a TSS reduction of ≥ 50% (TSS50)
- 21% of patients experienced improvements in bone marrow fibrosis grade
- 46% of patients demonstrated disease driver gene (JAK2 or CALR) variant allele frequency reductions.
- 29% of patients experienced bone marrow fibrosis improvement of ≥ 1 Grade at any time.
- Clinical outcomes were comparable between patients who had HMR mutations, or mutations in ≥ 3 genes, and those who did not (Table 2).
Table 2. Patient responses to navitoclax + ruxolitinib by genetic profile3
|
HMR, high mutational risk; SVR35, spleen volume reduction of ≥35%; TSS50, total symptom score reduction of ≥50%; VAF, variable allele frequency. |
||||
|
Week 24 results, % |
HMR |
Non-HMR |
≥ 3 genes mutated |
< 3 genes mutated |
|---|---|---|---|---|
|
SVR35 |
56 |
44 |
56 |
44 |
|
TSS50 |
83 |
17 |
67 |
33 |
|
-1/-2 fibrosis Grade reduction |
57 |
43 |
28 |
71 |
|
> 10% VAF reduction |
67 |
33 |
58 |
42 |
Safety
- Treatment-emergent adverse events (TEAEs) were observed in all patients, the most common were thrombocytopenia (88%), diarrhea (68%), and fatigue (62%).
- Grade ≥3 TEAEs were observed in 85% of patients, the most common were thrombocytopenia (53%), anemia (32%), and pneumonia (12%).
- No major bleeding events were associated with thrombocytopenia and it was managed with dose adjustments.
Inflammatory biomarker analysis
- Changes in spleen volume at Weeks 12 and 24 were shown to be directly correlated to changes in baseline levels of the MF-associated biomarkers:
- β2 microglobulin (B2M)
- Tissue inhibitor matrix metalloproteinase 1 (TIMP-1)
- Tumor necrosis factor receptor 2 (TNFR2)
- Vascular cell adhesion molecule 1 (VCAM-1)
- Myeloperoxidase (MPO)
- Changes in spleen volume at Week 12 were correlated with changes in baseline levels of insulin-like growth factor-binding protein 7 (IGFBP7), which was further associated with SVR at Week 24.
Conclusion
- Navitoclax + ruxolitinib demonstrated clinically meaningful outcomes in patients with MF who had received prior treatment with ruxolitinib.
- The presence of HMR mutations, or mutations in ≥ 3 genes, had no impact on SRV and improvement in TSS to navitoclax + ruxolitinib treatment.
- Cytokine analyses suggest that the drug combination may help to modulate MF-associated biomarker release.
Additional resources
Watch our interview below with Naveen Pemmaraju who discussed why adding navitoclax to ruxolitinib induces responses in patients with relapsed/refractory myelofibrosis.
Why does adding navitoclax to ruxolitinib induce responses in patients with R/R MF?
Ruxolitinib + KRT-2324
Dysregulation of the tumor suppressor protein, p53, is a hallmark of many malignancies including MF. Overexpression of the key negative regulator of p53, mouse double minute 2 homolog (MDM2), results in cancer cell proliferation and has been associated with driver mutations in MF. It has therefore been hypothesized that restoring p53 function and apoptosis via MDM2 inhibition may be a feasible treatment option for patients with MF.
KRT-232 is a first-in-class, orally available, selective, and potent MDM2 inhibitor which may have disease-modifying potential by targeting malignant stem cells and hematopoietic cells. In the phase IIa/IIb KRT-232-101 study (NCT03662126) conducted by Haifa Kathrin Al-Ali and coinvestigators, KRT-232 monotherapy demonstrated promising clinical efficacy and tolerability in patients with MF who had relapsed following, or were refractory to, JAKi therapy.
It has been suggested that ruxolitinib may augment the clinical activity of KRT-232. In a poster presentation from the 62nd ASH Meeting and Exposition, John Mascarenhas, Icahn School of Medicine at Mount Sinai, discussed the design of a prospective phase Ib/II study (NCT04485260) evaluating KRT-232 in combination with ruxolitinib in patients with MF with suboptimal responses to ruxolitinib. Below is a summary.
Study design
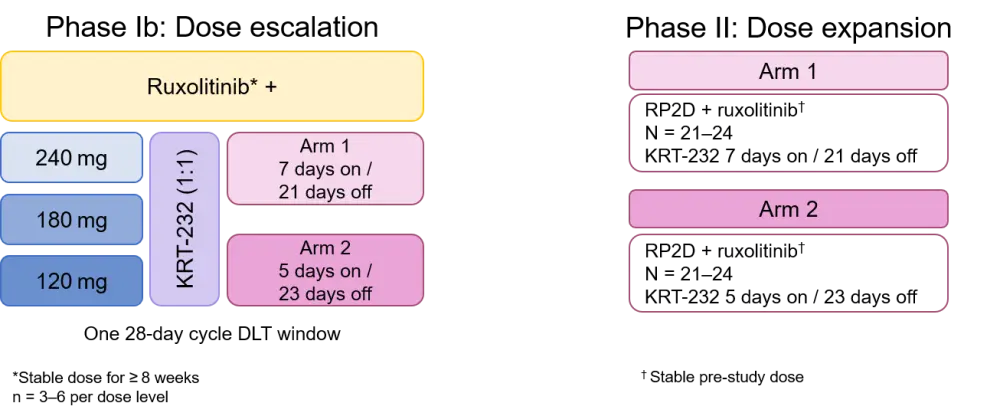
An open-label, multicenter, phase Ib/II study investigating the safety and efficacy of KRT-232 plus ruxolitinib in adult patients who meet the following enrollment criteria:
- Primary MF, post polycythemia vera MF (post-PV-MF), or post-essential thrombocythemia MF (post-ET-MF)
- Sub-optimal response to ruxolitinib following ≥ 18 weeks
- Receiving stable dose of ruxolitinib for ≥ 8 weeks prior to enrollment
- ECOG performance status of 0–2
The study will be conducted globally across 58 sites in North America, Europe, Asia, and Australia. In phase Ib, patients will be randomized 1:1 to arm 1 or 2, and dose escalation followed a 3 × 3 design whereby patients received treatment as outlined in Figure 2. The primary endpoint of phase Ib is to establish a recommended phase II dose of KRT-232 in combination with ruxolitinib. Secondary endpoints across the two phases include:
- SVR35
- Best change in TSS from baseline
- Duration of response
- Spleen size reduction
- Requirement for red blood cell transfusion
- ORR, OS, PFS, and leukemia-free survival
- Safety and tolerability
- Pharmacokinetics
Figure 2. Study schema for KRT-232 + ruxolitinib in patients with MF4
Additional resources
Why should we target MDM2 with small molecule inhibitor KRT-232 in patients with MF?
Overall conclusions
Both speakers highlighted the unmet clinical need for patients with MF who have suboptimal responses to, or relapse following, treatment with a JAKi, such as ruxolitinib.
The global effort to identify optimal therapies to manage patients with MF continues, and novel druggable targets and agents are being investigated in the MF setting. At ASH 2020, promising monotherapy data with momelotinib have been presented, which you can read more about here. For an overview of the novel agents for myeloproliferative neoplasms, including MF, read our review article here.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Haifa Kathrin Al-Ali
Haifa Kathrin Al-Ali Naveen Pemmaraju
Naveen Pemmaraju