All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Novel MPN therapies: single agents beyond Janus kinase inhibitors
Do you know... What is the overall response rate among patients treated with rusfertide in the REVIVE clinical trial?
Current goals for the treatment of myeloproliferative neoplasms (MPN) typically include reducing symptoms, splenomegaly, and the risk of thrombosis. However, many patients become treatment resistant or intolerant, highlighting the need for novel drugs targeting alternative pathways. During the 15th International Congress on MPN, Pemmaraju presented promising new drugs for MPN, highlighting agents beyond Janus kinase inhibitors (JAKis). Here, we summarize the key points.
Novel single agents1
Rusfertide
Polycythemia vera (PV) [JW1] is characterized by elevated levels of red blood cells, which can lead to thrombotic events. Rusfertide is an investigational hepcidin mimetic being evaluated in the phase II REVIVE study (NCT04057040) in patients with PV. Rusfertide offers a potential non-cytoreductive option to maintain normal hematocrit levels (<45%) and reduce the risk of thrombotic events.
- Rusfertide is delivered as a once-a-week, self-injectable dose
- REVIVE met its primary endpoint, with significant improvements in response rates vs placebo.
- The aim of the ongoing phase III VERIFY trial (NCT05210790) is to evaluate the safety and efficacy of rusfertide in subjects with PV while maintaining hematocrit and symptom control.
Overall response data from REVIVE-2 is presented in Figure 1.
Figure 1. REVIVE-2 ORRs*
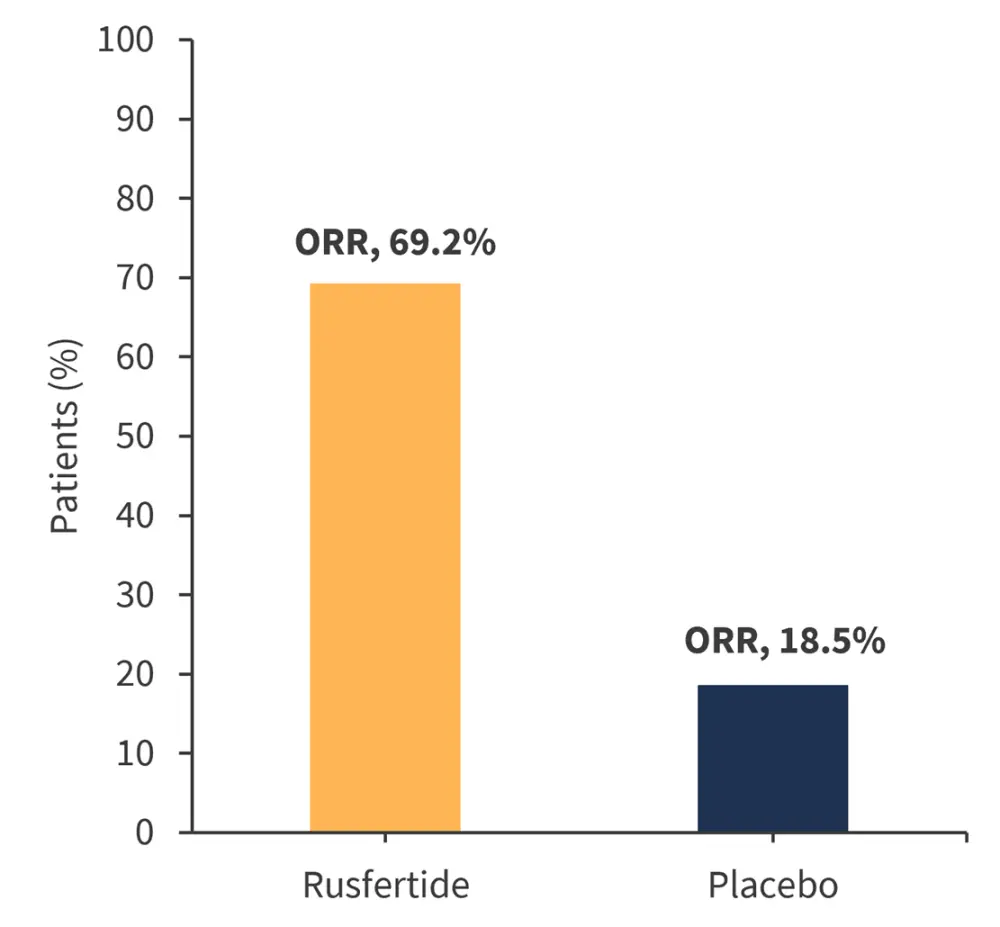
ORR, overall response rate.
*Adapted from Pemmaraju.1
Pelabresib
Pelabresib, a bromodomain and extra-terminal motif inhibitor, inhibits the function of bromodomain and extra-terminal motif proteins to suppress cytokine production, promote erythrocyte differentiation, and normal differentiation of megakaryocytes. Pelabresib is currently under investigation as a monotherapy and in combination with ruxolitinib as part of the phase II MANIFEST trial (NCT02158858).
At Week 24, significant improvements were observed in both ≥35% spleen volume reduction (68%) and total symptom score (56%).
Navitoclax
Navitoclax, an orally available inhibitor of B-cell lymphoma-2 and B-cell lymphoma-xL, is currently being investigated as part of the phase II REFINE trial (NCT03222609) in patients with myelofibrosis (MF). Navitoclax has demonstrated the potential to overcome JAKi resistance, presenting a feasible option for a significant proportion of patients who develop resistance.
The latest data from the REFINE trial of navitoclax in addition to ruxolitinib includes:
- Median overall survival (OS) at 2 years, not reached
- Survival estimates:
- 6 months, 100% (95% confidence interval [CI], 100–100)
- 12 months, 100% (95% CI, 100–100)
- 24 months, 84.7% (95% CI, 63.8–94.0)
LCL-161
LCL-161, a second mitochondrial-derived activator of caspases mimetic, aims to induce apoptosis in MF cells by binding to the cellular inhibitor of apoptosis protein-1 and the X-linked inhibitor of apoptosis protein.
The final results from a phase II trial investigating LCL-161 in treating patients with primary MF, post-PV MF, or post-essential thrombocytosis MF (NCT02098161) have been reported on the MPN Hub. LCL-161 treatment resulted in a response rate of 30% in patients with MF, with several responses in the anemic subgroup. Furthermore, LCL-161 treatment led to a median OS of 34 months, an important alternative endpoint for patients with MPN.
Tagraxofusp
Tagraxofusp, a drug approved by the U.S. Food and Drug Administration (FDA) for the treatment of blastic plasmacytoid dendritic cell neoplasms, targets the cluster of differentiation-123 pathway. A significant proportion of patients with MF overexpress cluster of differentiation-123, making it an appropriate marker in MF.
As a result, clinical trials of tagraxofusp have extended into MF, including a study of 39 patients in which a median OS of 26.6 months was observed and 49% of patients were alive at data cutoff.
Imetelstat
Imetelstat is a first-in-class inhibitor of telomerase activity currently under investigation for the treatment of advanced MF relapsed or refractory to JAKis (Figure 2). One of the primary aims for the use of imetelstat in MF is disease modification, with the phase III IMpactMF trial (NCT04576156) in progress in which OS is a primary endpoint.
Figure 2. Survival data following treatment with imetelstat in MF relapsed or refractory to JAKis*
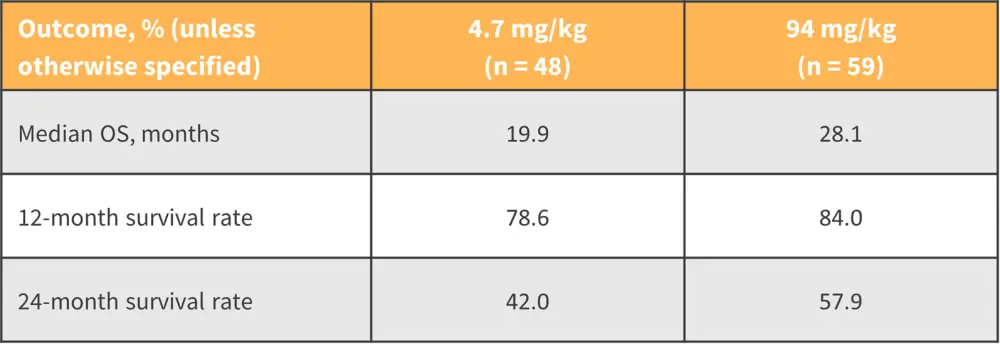
JAKi, Janus kinase inhibitor; MF, myelofibrosis; OS, overall survival.
*Adapted from Pemmaraju.1
TP-3654
TP-3654 is an investigational PIM1 (pim-1 proto-oncogene, serine/threonine kinase) inhibitor targeting a novel pathway in the treatment of MPN. Current phase I/II data (NCT04176198) suggests that TP-3654 is tolerable, with the most common adverse events being gastrointestinal. More data is required to determine the safety and efficacy of this therapy and the tolerability of gastrointestinal symptoms.
Preliminary signs of clinical activity include spleen volume reduction, symptom improvement, and broad cytokine reduction. Enrollment is ongoing for TP-3654 as monotherapy, and emerging data supports combination use with JAKis.
Zilurgisertib
Zilurgisertib is an oral activin receptor-like kinase-2 inhibitor currently under investigation as part of the phase I/II LIMBER-104 trial (NCT04455841) for its application to reduce anemia, including that which is caused by other MPN drugs. Initial data included 36 patients and indicated the drug may lead to improvements in anemia and transfusion dependence.
Conclusion
Several novel single agents are under investigation for the treatment of MPN. These include multiple alternatives targeting the Janus kinase/signal transducers and activators of transcription pathway, as well as those aimed at overcoming JAKi resistance. Consideration of alternative endpoints, such as disease modification, spleen volume reduction, OS, leukemia-free survival, progression-free survival, and bridge to transplant, is crucial in developing MPN management strategies. Early interventions, particularly in MF, may prove beneficial in modifying the bone marrow environment and effectively managing disease before it advances.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



