All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Managing anemia in patients with MF: Unmet needs and therapeutic options
Do you know... In the MOMENTUM extended follow-up study, what proportion of patients who received continuous momelotinib and who crossed over from initial treatment with danazol achieved transfusion independence, respectively?
Myelofibrosis (MF) is a Philadelphia chromosome-negative myeloproliferative neoplasm (MPN) characterized by abnormal megakaryocyte proliferation and reticulin or collagen fibrosis.1,2 The majority of patients diagnosed with MF (~90%) have activating JAK2, CALR, or MPL mutations, which lead to abnormal signaling resulting in the promotion of cell proliferation and survival and inflammatory pathway activation.1
Janus kinase (JAK) mutations are the most common mutations in MF, and JAK/STAT-mediated signaling pathway dysregulation leading to abnormal myeloproliferation and overproduction of inflammatory cytokines plays a key role in MF pathogenesis (Figure 1).1,3 JAK is therefore an important target for MF treatment, and several JAK inhibitors (JAKis) are now approved for the management of MF, including ruxolitinib, fedratinib, pacritinib, and momelotinib.3,4
Figure 1. Mode of action of JAK inhibitors in MF*
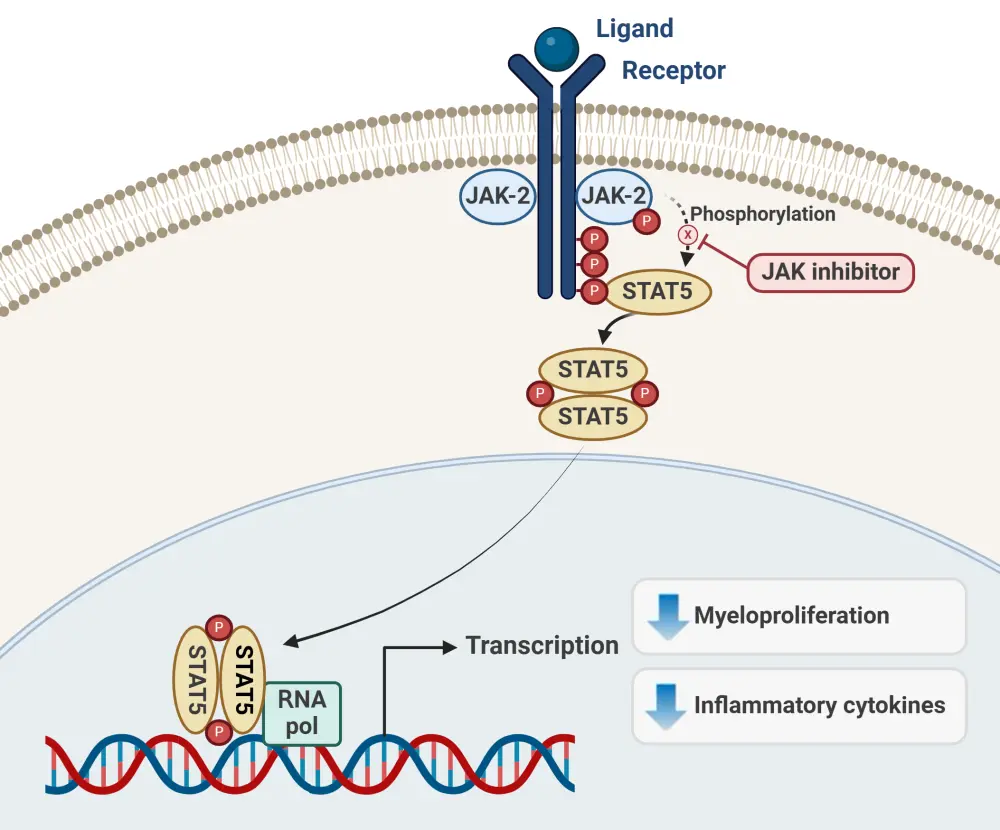
JAK, Janus kinase; MF, myelofibrosis.
*Data from Mascarenhas, et al.5 Created with BioRender.com.
Unmet needs
Anemia and transfusion dependence affect many patients with MF (Figure 2) and are associated with poor prognosis.6,7 MF-related anemia may be present at the time of MF diagnosis (potentially at severe levels of <8 g/dL hemoglobin) and tends to worsen over time as the disease progresses, or manifest due to JAKi treatment (treatment-related anemia).1
Figure 2. Anemia and transfusion dependence prevalence in patients diagnosed with MF*
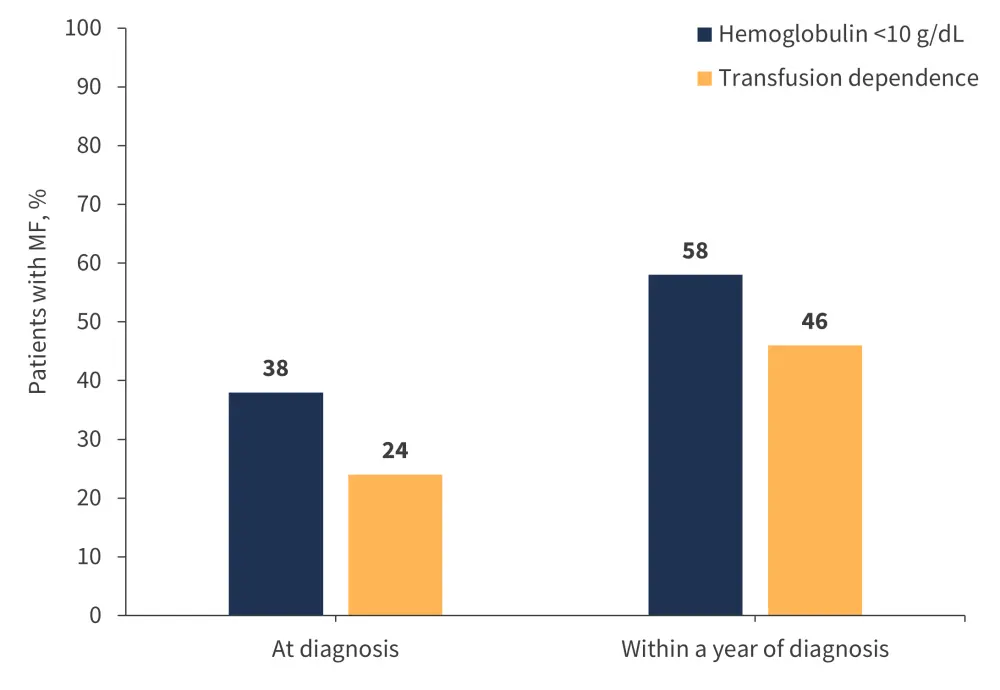
MF, myelofibrosis.
*Data from Tefferi et al.8
Anemia is associated with reduced survival in patients with MF, with one study demonstrating a median overall survival (OS) of 7.9 years in patients with MF and no anemia vs 2.1 years in patients with MF and severe anemia.9 As well as the association of MF-related anemia with a reduced survival, it also places a significant burden on patients, with fatigue, tiredness, and impaired ability to work and be productive experienced by ~90% of patients.1,10
Current treatment strategies for MF-related anemia include red blood cell (RBC) transfusions, erythropoiesis-stimulating agents, androgens, steroids, splenectomy, immunomodulatory drugs, and JAKi.4 All these options demonstrate limited efficacy and response durability, and produce multiple side effects that impact patient quality of life and outcomes.4 Whilst currently approved JAKi therapies for MF offer clinical benefits, there remain limitations to their use.2,4 For example, ruxolitinib and fedratinib can worsen anemia and thrombocytopenia, potentially leading to treatment interruptions and dose reductions and thereby limiting treatment efficacy.2,11 As such, there is a high unmet need for safe and effective treatments for patients with MF-related anemia.2,4
Anemia in MF: Insights from phase III trials and extended follow-up studies
Ruxolitinib was the first JAKi approved for the treatment of MF based on efficacy and safety findings from the phase III COMFORT-I (NCT00952289) and COMFORT-II (NCT00934544) trials, and is currently indicated for patients diagnosed with intermediate- or high-risk MF.12 However, both anemia and thrombocytopenia arose in ruxolitinib-treated patients in the COMFORT studies, as well as in the phase IIIb expanded-access JUMP (NCT01493414) trial.4 A post hoc analysis of pooled data from the COMFORT trials found that 61% of ruxolitinib-treated patients without anemia at baseline developed anemia and that 69% of ruxolitinib-treated patients with anemia experienced worsening anemia.4 Anemia thereby presents a treatment limitation for ruxolitinib and contributes to treatment discontinuation in both clinical trials and in the real-world world setting.4,11
A study investigating predictors of early ruxolitinib discontinuation and death in 889 patients with MF found that 50% to 70% of patients discontinue ruxolitinib within 3–5 years.13 A sub analysis of the study focused on 410 patients who were alive on ruxolitinib after ≥5 years from ruxolitinib start, alive off ruxolitinib after ≥5 years, and who died while on ruxolitinib after <5 years found that hemoglobin <10 g/dL (hazard ratio [HR], 1.99; 95% confidence interval [CI], 1.37–2.91; p < 0.001) and transfusion dependence (HR, 2.05; 95% CI, 1.32–3.18; p = 0.001) were associated with a higher probability of ruxolitinib discontinuation within 5 years of starting therapy.13 High rates of treatment-emergent anemia were observed (Table 1), contributing to early discontinuations and thereby reduced OS outcomes. In an analysis of clinical practice findings from 20 hematology centers, 27.5% of patients discontinued ruxolitinib due to treatment-related adverse events, with 10.5% of discontinuations due specifically to treatment-related anemia.4
Table 1. Ruxolitinib treatment-emergent anemia in patients with MF (n = 410)*
|
MF, myelofibrosis. |
|||
|
|
Follow-up ≥5 years |
Follow-up <5 years |
|
|---|---|---|---|
|
Long-term ruxolitinib† |
Short-term ruxolitinib‡ |
Early death on ruxolitinib |
|
|
Treatment-emergent anemia, % |
|
|
|
|
At 3 months |
64.2 |
67.8 |
53.8 |
|
At 6 months |
49.0 |
48.9 |
33.9 |
Fedratinib was subsequently approved for the treatment of MF was based on findings from the phase III JAKARTA (NCT01437787) and JAKARTA2 (NCT01523171) trials.4 The most common hematologic adverse events (AEs) in these trials were anemia and thrombocytopenia, and as with ruxolitinib, fedratinib does not improve MF-associated anemia.4 Anemia is a commonly observed treatment-emergent adverse event with fedratinib, with new or worsening Grade 3 anemia in around a third of patients.14 Pacritinib was approved later, based on findings from the phase III PERSIST-1 (NCT01773187) and PERSIST-2 (NCT02055781) trials. Patients receiving 200 mg pacritinib twice daily in the PERSIST 2-trial experienced hemoglobin improvements and reduced transfusion requirements, but also experienced cytopenia AEs at higher rates than patients receiving best available therapy (BAT; anemia, 24% vs 15%; thrombocytopenia, 34% vs 23%).15 Pacritinib is approved by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) for patients with MF and platelet counts <50 × 109/L, with ruxolitinib and fedratinib both contraindicated in patients with platelet counts <50 × 109/L.7
Luspatercept is an erythroid maturation agent currently under investigation for the treatment of patients with MF and anemia with and without RBC transfusion dependence and concurrent JAKi therapy.16 Results from the phase II ACE-536-MF-001 (NCT03194542) trial support luspatercept as a feasible option for the treatment of anemia in patients with MF, reducing the need for transfusions and potentially allowing maintenance of JAKi therapy.16 A phase III trial of luspatercept in transfusion-dependent patients with MF receiving concomitant JAKi therapy is underway (INDEPENDENCE [NCT04717414] trial).16
Momelotinib
Momelotinib is an ATP-competitive small molecule inhibitor of JAK proteins (including JAK1, JAK2, JAK3), TYK2, and activin A receptor type 1 (ACVR1).17 This dual mechanism of action leads to reduced hepcidin expression and increased iron availability for erythropoiesis (Figure 3).17,18
Figure 3. Momelotinib mode of action*
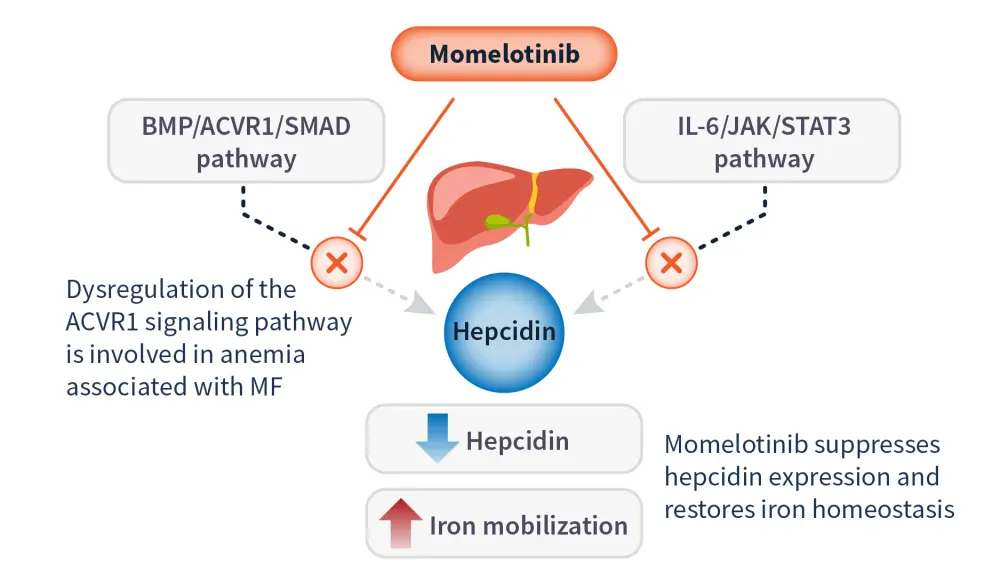
ACVR1, activin A receptor type 1; BMP, bone morphogenetic protein; IL, interleukin; JAK, Janus kinase; MF, myelofibrosis; STAT, signal transducer and activator of transcription.
*Data from Chifoides, et al.18
Momelotinib was approved by the FDA in 2023 and by the EMA in 2024 for use in anemic patients with high/intermediate risk MF, including primary and secondary MF (post-polycythemia vera or post-essential thrombocythemia).17 The approvals were granted on the basis of findings from the phase III SIMPLIFY-1 (NCT01969838) and SIMPLIFY-2 (NCT02101268) trials, which demonstrated robust OS outcomes in both JAKi-naïve and JAKi-treated patients with MF that did not discriminate according to initial therapy.19,20 The phase III MOMENTUM (NCT04173494) trial confirmed the differentiated clinical benefits of momelotinib vs danazol in symptomatic and anemic patients previously treated with a JAKi therapy for MF.21 The MPN Hub previously reported on an integrated long-term pooled analysis of the SIMPLIFY-1, SIMPLIFY-2, and MOMENTUM trials, covering efficacy and safety data of momelotinib in MF from early (JAKi-naïve) to late (JAKi-experienced) disease stages.2
In an updated analysis of the MOMENTUM trial, Gerds et al.22 reported the duration of Week 24 responses and new responses with momelotinib through Week 48. In this analysis, a total of 195 patients were randomized to momelotinib (n = 130) or danazol (n = 65), with 72% and 63% of patients, respectively, entering the momelotinib open-label extension period.22 At Week 24, transfusion independence response rates were 30% with momelotinib vs 20% with danazol.22 At Week 48, 57% of patients who continued receiving momelotinib and 60% of patients who crossed over from the danazol group achieved transfusion independence, with 52% and 56% from the momelotinib and crossover groups, respectively, achieving transfusion independence at any time during the open label period by Week 48 (Figure 4).22 Transfusion independence was maintained in 87% of Week 24 responders receiving continuous momelotinib.22
Figure 4. Transfusion independence response rates in the MOMENTUM trial*
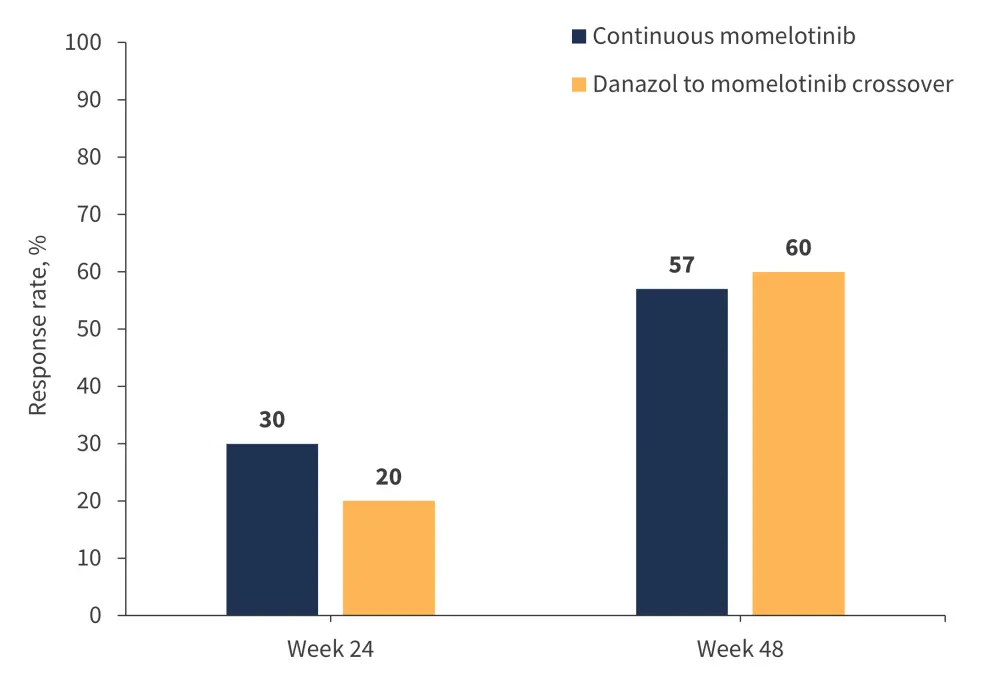
*Data from Gerds, et al.22
Importantly, momelotinib provided improved total symptom score and spleen volume reduction endpoints, as well as improved transfusion endpoints, highlighting benefits for both MF and anemia (Table 2).6,22
Table 2. Response rates at Weeks 24 and 48 of the MOMENTUM trial with extended follow-up*
|
SVR, spleen volume reduction; TSS, total symptom score. |
||||
|
Response, % |
Continuous momelotinib |
Danazol to momelotinib crossover |
||
|---|---|---|---|---|
|
Week 24† |
Week 48‡ |
Week 24† (danazol) |
Week 48‡ (momelotinib) |
|
|
TSS response |
25 |
45 |
9 |
50 |
|
SVR ≥25% |
39 |
64 |
6 |
37 |
|
SVR ≥35% |
22 |
43 |
3 |
13 |
Both momelotinib and danazol led to rapid improvements in mean hemoglobin levels during the randomized period, and these improvements were maintained through Week 48 with open-label momelotinib.22 Patients crossing over from danazol may have derived additional anemia benefits from momelotinib, as evidenced by increases in mean hemoglobin levels and new transfusion independence responses arising after Week 24 in this group.22
Momelotinib is the only JAKi to have demonstrated consistent and prospectively evaluated anemia benefits across phase III trials, and this updated analysis provides further evidence of the long-term reduction in anemia burden achievable with momelotinib treatment.6 An exploratory analysis that evaluated survival adjusted for treatment crossover throughout the entire MOMENTUM trial period using a rank-preserving structural failure time (RPSFT) model to estimate the OS and leukemia-free survival that might have been observed without crossover was recently published by Gupta et al.23 The findings adjusted for treatment crossover are consistent with the original survival analysis, demonstrating prolonged OS and leukemia-free survival in patients initially randomized to momelotinib vs those initially randomized to danazol, with HRs in favor of momelotinib.23
In an analysis of 72 patients who were JAKi-naïve and anemic prior to momelotinib treatment, 44% achieved an anemia response (with a median duration of response of ~20 months).17 In a multivariable analysis of outcomes in this patient population, anemia responses positively affected survival outcomes (median 3.8 years with anemia response vs 2.8 years without response).17 The positive impact of anemia response on survival outcomes were also confirmed in transfusion-dependent patients (median, 3.7 years vs 1.9 years, respectively, with a 10-year survival of 8% vs 0%).17 Upcoming data from an extended access trial of momelotinib in adults with MF (NCT03441113) will provide further insights into long-term outcomes.6,22 Trials investigating JAKi treatment in combination with an agent to address anemia (such as the phase III INDEPENDENCE (NCT04717414) trial, which includes combined dosing of a JAK2i with luspatercept) will provide important insights into the potential for combination therapies to address anemia in patients with MF.4
Findings from the SIMPLIFY-1 trial demonstrated the anemia benefits of momelotinib vs ruxolitinib, with significantly superior rates of transfusion independence by Week 24 (Figure 5) and significantly superior improvements in mean hemoglobin levels by Week 24, which continued improving through Week 36.19,24
Figure 5. Transfusion independence rates with momelotinib vs ruxolitinib in the SIMPLIFY-1 trial*
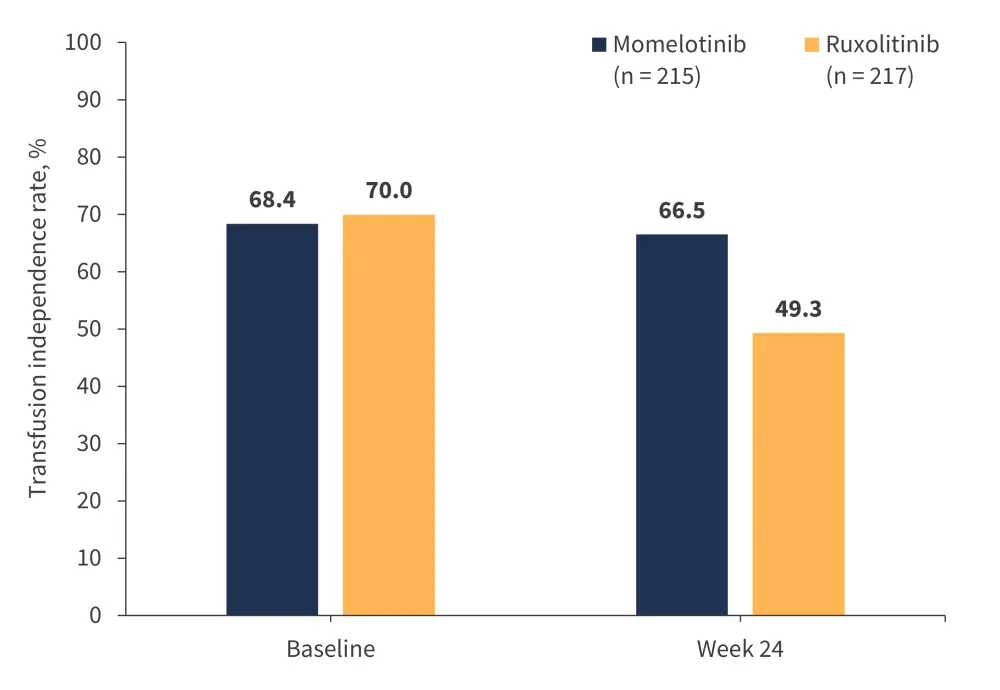
*Adapted from Mesa, et al.19
In a post hoc subgroup analysis of the phase III SIMPLIFY-2 study (momelotinib vs BAT; 88.5% continued ruxolitinib) in JAKi-experienced patients with MF (n = 156), anemia-related benefits, including mean hemoglobin levels over time and transfusion independence rates at Week 24, were higher with momelotinib vs BAT in both patients who were non-transfusion independent at baseline and those with baseline hemoglobin of <100 g/L.25 The analysis provided evidence that in patients with moderate-to-severe anemia and/or those who were transfusion dependent, switching to momelotinib improved outcomes when compared to continuing with ruxolitinib and providing supportive therapies for anemia.25
An integrated analysis of phase III randomized controlled trials in which patients with MF received momelotinib (N = 725) characterized the overall safety profile of momelotinib by summarizing AEs and survival across the three trials.2 The analyses showed that the incidence of most toxicities, including hematologic AEs, decreased over time even with the continuation of momelotinib dosing.2 Momelotinib dose intensity was maintained throughout treatment duration (with a median relative dose intensity of 97.3%), allowing an effective therapeutic drug exposure to be preserved.2 These findings highlight the benefit of momelotinib in patients with anemia compared with other JAKi treatments, which often require dose modifications.2
Current treatment guidance for anemia
Guidelines are being updated to reflect developments in the treatment of anemia in MF. Momelotinib was recommended for the treatment of patients with MF and anemia by a 2024 global consensus group, which provided updated recommendations on the management of MF in routine clinical practice, with a focus on patients with cytopenias.26 In addition, the most recent British Society for Haematology (BSH) Guidelines recommend momelotinib for consideration for patients with MF and anemia in all lines of therapy.7 The BSH suggested algorithms for the management of MF-associated anemia are outlined in Figure 6. Expert opinions such as this recent interview with Jean-Jacques Kiladjian on the MPN Hub also express that momelotinib has potential to fill an unmet need in both the first and subsequent lines of therapy settings.
Figure 6. BSH suggested algorithm for the management of MF-associated anemia*
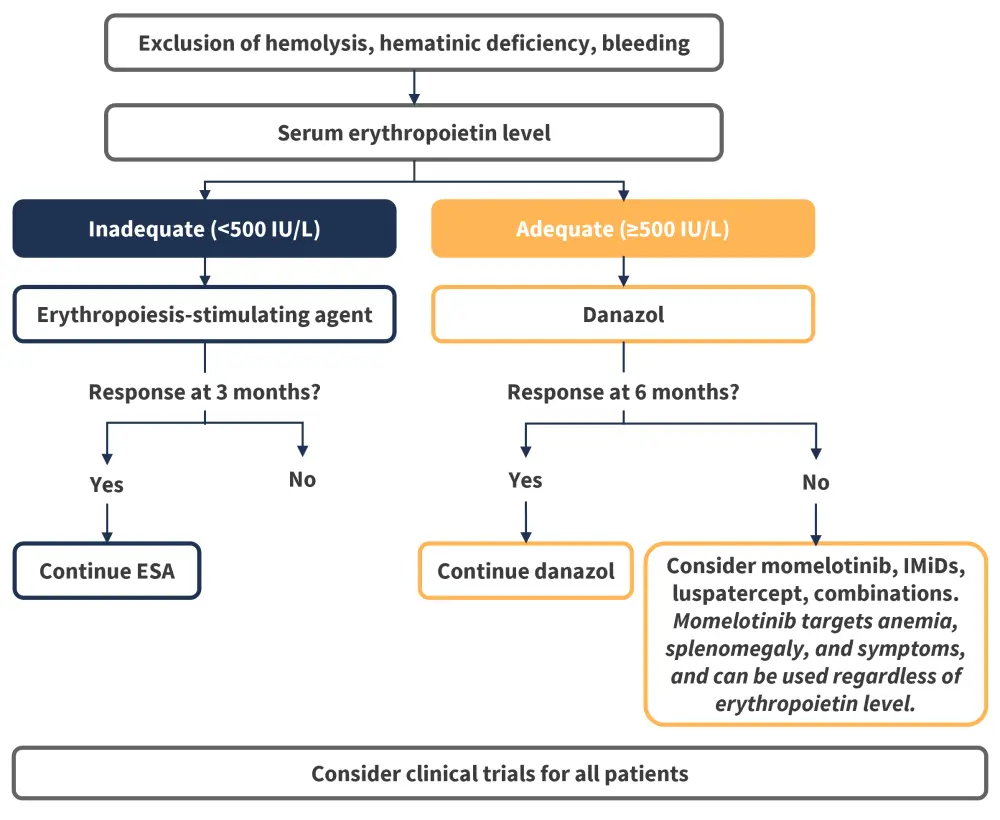
BSH, British Society for Haematology; IMiD, immunomodulatory drug; MF, myelofibrosis.
*Data from McLornan, et al.7
The most recent National Comprehensive Cancer Network (NCCN) guidelines for the management of MF-associated anemia recommend clinical trial enrollment as the preferred option for all patients, with momelotinib as a co-preferred option for patients with uncontrolled symptomatic splenomegaly and/or constitutional symptoms.27 For patients without symptomatic splenomegaly and/or constitutional symptoms, other recommendations include luspatercept, danazol, momelotinib, and pacritinib.27 For patients with anemia and symptomatic splenomegaly and/or constitutional symptoms, recommendations include ruxolitinib combinations as well as luspatercept, danazol, momelotinib, and pacritinib in certain circumstances.27
Emerging treatment options
Further agents targeting different mechanisms of action are in development for the treatment of anemia in MF based on advances in understanding of the pathogenesis of MF-related anemia (Table 3). Early data indicate clinical improvements in anemia and transfusion dependence and ongoing trials will further evaluate the effectiveness of these therapies.
Table 3. Emerging treatments for anemia in myelofibrosis*
|
ACTRIIA, activin receptor IIA; BET, bromodomain and extra-terminal domain; BM, bone marrow; HSPC, hematopoietic stem and progenitor cell; JAK, Janus kinase; MoA, mechanism of action; LOXL2, lysyl oxidase-like 2; RBC, red blood cell; TGF-β, transforming growth factor beta. |
||
|
Therapy class |
Rationale/MoA |
Examples |
|---|---|---|
|
TGF-β ligand traps |
Disruption of activated TGF-β/SMAD signaling can prevent apoptosis of erythroblasts and promote erythroblast maturation |
Luspatercept (erythroid maturation agent) KER-050 (modified ActRIIA ligand trap) |
|
Epigenetic modulators |
BET inhibition can reduce inflammatory responses responsible for disrupting the BM microenvironment and contributing to anemia |
Pelabresib (BET inhibitor) |
|
Antifibrotic agents |
Addressing BM fibrosis can normalize the BM microenvironment to enhance effective erythropoiesis and prevent further erythropoietic tissue expulsion |
|
|
Telomerase inhibitors |
Telomerase inhibition in malignant HSPCs contributes to their selective elimination, allowing for normal RBC production |
Imetelstat (telomerase inhibitor) |
|
BcL-2/BcL-xL inhibitors |
Inhibition of pro-apoptotic factors reduces apoptosis of maturing RBCs |
Navitoclax (Bcl-2/Bcl-xL/Bcl-w inhibitor) |
Conclusion
Anemia represents a key unmet need for patients with MF, contributing to decreased quality of life and survival, highlighting the need for new treatments.1,4 Novel treatment options for this patient population are an important area of focus for ongoing research and clinical practice.11 Emerging clinical trial data evaluating therapies for MF-related anemia have shown positive results. Phase II results support luspatercept as a feasible option for the treatment of anemia in patients with MF. Moreover, momelotinib, a recently approved JAKi that reduces hepcidin expression and increases iron availability for erythropoiesis, offers a safe and effective long-term treatment for patients with MF and anemia with significant anemia response rates.4,11,21,29 Data across phase III clinical trials support the treatment of patients with anemia with momelotinib in the first-line setting, and highlight the importance of considering pre-existing anemia in patients with MF before making a treatment decision.2,25,26 Ongoing trials of novel agents, either alone or in combination with JAKi therapy may lead to new treatment options for patients with anemia and MF in the future.
This educational resource is independently supported by GSK. All content was developed by SES in collaboration with an expert steering committee; funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




