All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Momelotinib for the treatment of MF: Long-term data
Do you know... What percentage of patients achieved transfusion independence at Week 48 with continuous momelotinib treatment in the updated analysis of the phase III MOMENTUM trial?
Myelofibrosis (MF) is a Philadelphia chromosome-negative myeloproliferative neoplasm characterized by splenomegaly, anemia, and thrombocytopenia, and symptoms include fatigue, bone pain, cachexia, night sweats, and fever, which negatively impact patients’ quality of life.1 Janus kinase (JAK)-mediated signaling pathway dysregulation leading to abnormal myeloproliferation and overproduction of inflammatory cytokines plays a key role in MF pathogenesis, and several JAK inhibitors (JAKis) are now approved for the management of MF.1,2 In chronological order of approval, approved JAKi agents include ruxolitinib, fedratinib, momelotinib, and pacritinib.
Although ruxolitinib, fedratinib, and pacritinib offer clinical benefits, there remain limitations to their use.1 For example, ruxolitinib and fedratinib can worsen anemia and thrombocytopenia, potentially leading to treatment interruptions and dose reductions and thereby limiting treatment efficacy.1 As such, there is an unmet need for safe and effective treatments for patients with MF who are anemic or unable to continue receiving currently approved JAKis.1 In this article, we explore the long-term efficacy and safety outcomes of momelotinib for the treatment MF.
Momelotinib for the treatment of MF
Momelotinib is a first-in-class oral inhibitor of JAK1, JAK2, and activin A receptor type 1 (ACVR1), which has demonstrated improvements in splenomegaly, constitutional symptoms, and anemia in both JAKi-naive and previously treated patients with MF in several clinical trials.1,2 In the phase III SIMPLIFY-1 (NCT01969838) and SIMPLIFY-2(NCT02101268) trials, momelotinib demonstrated robust overall survival (OS) outcomes in both JAKi-naïve and JAKi-treated patients with MF that did not discriminate according to initial therapy.3,4 The phase III MOMENTUM trial(NCT04173494) confirmed the differentiated clinical benefits of momelotinib vs danazol in symptomatic and anemic patients previously treated with a JAKi therapy for MF.5
Momelotinib long-term survival and safety in MF
The MPN Hub previously reported on an integrated long-term pooled analysis of the SIMPLIFY-1, SIMPLIFY-2, and MOMENTUM trials, covering efficacy and safety data in MF from early (JAKi-naïve) to late (JAKi-experienced) disease stages.1 With a median follow-up of 3 years in the pooled population from these trials, the median OS was not reached, with survival probabilities over time outlined in Figure 1.1
Figure 1. Survival probabilities in the pooled momelotinib-treated population from SIMLIFY-1, SIMPLIFY 2, and MOMENTUM (N = 725)*
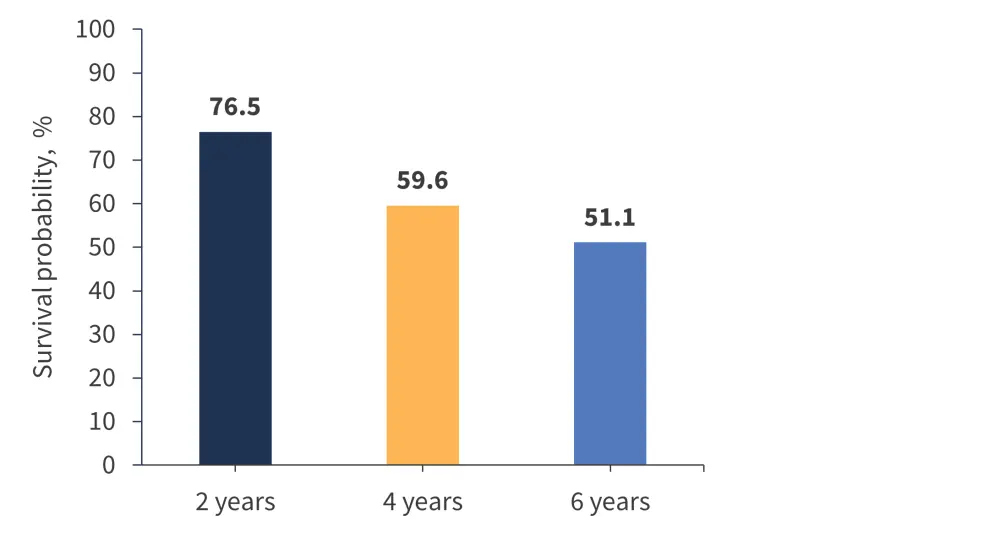
*Data from Verstovsek at al.1
In this pooled analysis, a total of 725 patients with MF received momelotinib treatment, with a median treatment exposure of 11.3 months (range, 0.1–90.4 months), and 12% of patients remained on therapy for ≥5 years.1 Most adverse events (AEs) were of Grade 1 or 2 severity and did not worsen over time.1 There was no time trend suggesting late onset or cumulative toxicity with continued treatment, and the incidence of most toxicities decreased over time despite continued dose intensity and administration.1 Overall, these results highlighted that continued momelotinib treatment is well tolerated in most patients and represents a positive risk–benefit balance in patients with intermediate and high-risk MF.1
MOMENTUM updated analysis through Week 48
The primary endpoint for the MOMENTUM trial (Myelofibrosis Symptom Assessment Form Total Symptom Score [MFSAF TSS] response rate at Week 24) was met, with a significantly higher proportion of patients reporting a ≥50% reduction in TSS in the momelotinib group compared with the danazol group (25% vs 9%, respectively).5 In an updated analysis of the trial, Gerds et al.6 reported duration of Week 24 responses and new responses with momelotinib through Week 48. In this analysis, a total of 195 patients were randomized to momelotinib (n = 130) or danazol (n = 65), with 72% and 63% of patients, respectively, entering the momelotinib open-label extension period.6
At Week 48, 45% of 67 TSS-evaluable patients who continued momelotinib treatment and 50% of 30 TSS-evaluable patients who crossed over from the danazol group were responders.6 At any time during the open-label period, 61% of 75 evaluable patients in the continuous momelotinib group and 59% of the 32 patients who crossed over from the danazol group were TSS responders by Week 48 (Table 1).
Table 1. Response rates at Weeks 24 and 48*
|
Response, % |
Continuous momelotinib |
Danazol to momelotinib crossover |
||
|
Week 24† |
Week 48‡ |
Week 24† (danazol) |
Week 48‡(momelotinib) |
|
|
SVR ≥25% |
39 |
64 |
6 |
37 |
|
SVR ≥35% |
22 |
43 |
3 |
13 |
|
Transfusion independence |
30 |
57 |
20 |
60 |
|
TSS response |
25 |
45 |
9 |
50 |
|
SVR, spleen volume reduction; TSS, Total Symptom Score. |
||||
At Week 24, transfusion independence response rates were 30% with momelotinib and 20% with danazol.6 At Week 48, 57% of patients who continued receiving momelotinib and 60% of patients who crossed over from the danazol group achieved transfusion independence, with 52% and 56% from the momelotinib and crossover groups, respectively, achieving transfusion independence at any time during the open label period by Week 48.6 The median duration of Week 24 transfusion independence response was not reached in either group.6 Transfusion independence was maintained in 87% of Week 24 responders receiving continuous momelotinib.6
Both momelotinib and danazol led to rapid improvements in mean hemoglobin levels during the randomized period, and these improvements were maintained through Week 48 with open-label momelotinib.6 Patients crossing over from danazol may have derived additional anemia benefits from momelotinib, as evidenced by increases in mean hemoglobin levels and new transfusion independence responses arising after Week 24 in this group. Momelotinib is the only JAKi to have demonstrated consistent and prospectively evaluated anemia benefits across phase III trials, and this updated analysis provides further evidence of the long-term reduction in anemia burden achievable with momelotinib treatment.6
A total of 171 patients treated with momelotinib were evaluable for safety over the entire study period, comprising 130 patients from the momelotinib group and 41 patients who crossed over from the danazol group.6 With extended follow-up, no new safety signals were observed.6 The most common non-hematologic treatment-emergent AEs (TEAEs); Grade 3–4, serious, and fatal TEAEs; infections; and AEs of special interest in patients treated with momelotinib over the entire study duration are summarized in Table 2.
Table 2. Summary of TEAEs in the MOMENTUM long-term follow-up.*
|
TEAE, % |
Momelotinib-treated patients |
|
Non-hematological events |
|
|
Diarrhea |
26 |
|
Asthenia |
16 |
|
Grade 3–4 TEAEs |
|
|
Thrombocytopenia |
19 |
|
Anemia |
11 |
|
Serious TEAEs |
46 |
|
Fatal TEAEs |
18 |
|
Infections |
47 |
|
AEs of interest with JAKis† |
|
|
AML or leukemic transformation |
4 |
|
Major adverse cardiovascular events |
3 |
|
Peripheral sensory neuropathy |
5 |
|
AML, acute myeloid leukemia; TEAE, treatment-emergent adverse event. |
|
These updated analyses demonstrate the durability of transfusion independence and hemoglobin level improvements through Week 48.6
Long-term survival adjusted for treatment crossover
An exploratory analysis that evaluated survival adjusted for treatment crossover over the entire MOMENTUM trial period using a rank-preserving structural failure time (RPSFT) model to estimate the OS and leukemia-free survival (LFS) that might have been observed without crossover was recently published by Gupta et al.7 At the cutoff date of December 29, 2022, 29% and 31% of deaths occurred in the momelotinib and danazol arms, respectively.7 The risk of death was reduced by 11% (hazard ratio [HR], 0.89) with momelotinib vs danazol with no crossover adjustment and by 22% (HR, 0.78) and 13% (HR, 0.87) using the RPSFT model with and without recensoring, respectively.7 By December 2022, LFS events had occurred in 31% and 34% of patients in the momelotinib and danazol arms, respectively.7 At this data cutoff, the risk of an LFS event was reduced by 20% (HR, 0.80) with momelotinib vs danazol with no crossover adjustment and by 36% (HR, 0.64) and 23% (HR, 0.77) using the RPSFT model with and without recensoring, respectively. These findings adjusted for treatment crossover are consistent with the original survival analysis, demonstrating prolonged OS and LFS in patients initially randomized to momelotinib vs those initially randomized to danazol, with HRs in favor of momelotinib. An update to this analysis was presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, with a data cutoff of July 2023.8 With this additional follow-up, the risk of death was similar across treatments arms (HR, 0.99) in both the unadjusted and RPFST-adjusted analyses, with an OS of 37% in both groups.8However, limited sample size and event count preclude definitive conclusions at this cutoff.
Conclusion
Momelotinib provides an effective long-term management option for patients with MF, resulting in long-term survival benefits and reductions in splenomegaly, constitutional symptoms, and anemia in a high proportion of patients, with a tolerable safety profile.1,6,7 Momelotinib may offer an important treatment option for patients with MF who are anemic or unable to receive currently approved JAKis, potentially bridging a gap in a population with an unmet need.1 Upcoming data from an extended access trial (NCT03441113) of momelotinib in adults with MF will provide further insights into long-term safety and efficacy.6,7
Your opinion matters
As a result of this content, I commit to reviewing the latest clinical data for momelotinib to help inform my treatment choices for patients with myelofibrosis.
This educational resource is independently supported by GSK. All content was developed by SES in collaboration with an expert steering committee. Funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content





