All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Visual abstract | FREEDOM2: Fedratinib vs BAT in in patients with MF previously treated with ruxolitinib
Do you know... What difference was observed in the proportion of patients reaching SVR35 at end of Cycle 6 (EOC6) with fedratinib vs BAT in the FREEDOM2 trial?
Myelofibrosis (MF) is a group of myeloproliferative neoplasms (MPN) that includes primary MF and secondary MF (post-polycythemia vera [PV] and post-essential thrombocythemia [ET] MF).1 MF is characterized by abnormal clonal expansion of hematopoietic stem cells resulting in bone marrow fibrosis. Patients can be stratified into low, intermediate-1, intermediate-2, and high-risk categories, which correlate with patient prognoses.1
Currently approved treatment options for patients with higher risk MF include hematopoietic stem cell transplantation and Janus kinase inhibitors, including ruxolitinib, fedratinib, momelotinib, and pacritinib.1 Whilst ruxolitinib offers an effective frontline treatment for intermediate- and high-risk MF, ∼75% of patients discontinue treatment within 5 years due to disease progression or ruxolitinib intolerance, with poor survival rates following discontinuation. As such, treatments for patients with MF previously treated with ruxolitinib are a high unmet need.1
Fedratinib was approved for the treatment of adults with intermediate-2 and high-risk primary MF, post-PV MF, and post-ET MF based on efficacy data from the phase III JAKARTA trial (NCT01437787).1 Here, we present a visual abstract outlining the trial design and summarizing key efficacy and safety data from the phase III FREEDOM2 trial (NCT03952039) investigating fedratinib vs best available therapy in patients with MF previously treated with ruxolitinib (N = 201) published in Lancet Haematology by Claire Harrison et al.1
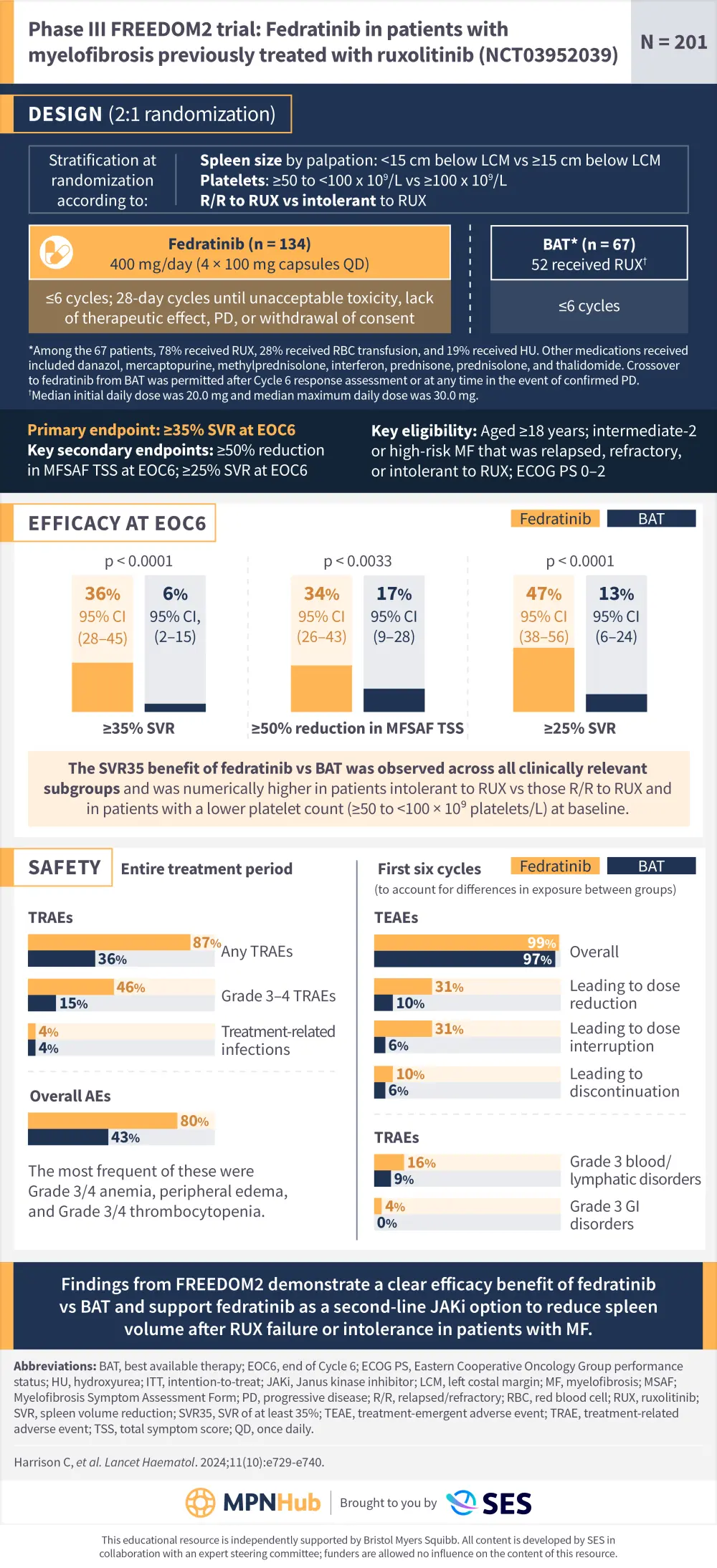
This educational resource is independently supported by Bristol Myers Squibb. All content was developed by SES in collaboration with an expert steering committee. Funders were allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content






