All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the MPN Advocates Network.
The mpn Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mpn Hub cannot guarantee the accuracy of translated content. The mpn and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The MPN Hub is an independent medical education platform, sponsored by AOP Health, GSK, Sumitomo Pharma, and supported through educational grants from Bristol Myers Squibb and Incyte. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View MPN content recommended for you
Novel non-JAK inhibitors for MF: Updates from SOHO 2023
Do you know... What is the therapeutic target for pelabresib, an investigational drug for myelofibrosis?
Inhibiting the Janus kinase (JAK) pathway is considered the hallmark myelofibrosis (MF) treatment; however, treatment discontinuation due to adverse events presents an ongoing challenge. After JAK inhibition (JAKi) failure, therapeutic options are limited and there is a need for therapies beyond JAKis. Several such therapies are in clinical development, have acceptable safety, and demonstrate attenuation of key symptoms including splenomegaly.
The MPN Hub previously published an article summarizing updates on non-JAKi for MF. Here, we provide an overview of the latest updates on novel non-JAKi for MF, presented by Mascarenhas during the Society of Hematologic Oncology (SOHO) 2023 Annual Meeting.
Pelabresib + ruxolitinib1
Pelabresib is a bromodomain and extra-terminal protein inhibitor (BETi) currently under investigation in the phase II MANIFEST trial (NCT02158858) in patients with MF and essential thrombocytopenia (ET) across multiple arms. Arm 3 evaluates first-line pelabresib plus ruxolitinib in patients with no prior JAKi treatment.
Efficacy and safety
- Among 84 patients treated, 68% achieved a spleen volume reduction ≥35% (SVR35) at Week 24.
- At data cut off, responses were durable in 47 out of 67 patients (70%).
- A ≥50% reduction in total symptoms score (TSS50) was seen in 56% (46/82) at Week 24, and 83% (68/82) at any time.
- Hemoglobin improvement was observed over time, with a hemoglobin response reported in 28% (22/79) of the non-transplant dependent cohort.
- Bone marrow fibrosis (BMF) was improved by ≥1 Grade in 27% (17/63) of patients at Week 24 and in 40% at any time.
- A ≥20% reduction in JAK2 V617F variant allele frequency (VAF) burden was achieved in 38% (18/47) of patients in the first 6 months of treatment; this overlapped with BMF improvement and SVR35 in eight patients, TSS50 in five patients, and hemoglobin responses in five patients.
Overall, this combination was well-tolerated, with minimal gastrointestinal toxicity reported.
- Serious adverse events included anemia, pyrexia, and COVID-19 in three patients each;
- multi-organ dysfunction syndrome, pneumonia, respiratory tract infection, urinary tract infection, fall, and respiratory failure in two patients each;
- treatment related adverse events leading to pelabresib discontinuation occurred in 12 patients; and
- Grade 5 treatment emergent AEs were reported in seven patients.
A matching adjusted indirect comparison analysis revealed a higher SVR and TSS for pelabresib plus ruxolitinib compared with ruxolitinib, fedratinib, and momelotinib across all trials. The randomized phase III MANIFEST-2 trial investigating pelabresib + ruxolitinib versus placebo + ruxolitinib in JAKi-naïve patients with MF is currently ongoing (NCT04603495).
Navitoclax + ruxolitinib1
Navitoclax, a B-cell lymphoma-2 (Bcl-2) inhibitor, plus ruxolitinib has been evaluated in the phase II REFINE trial (NCT03222609) in JAKi-naïve patients with MF. This combination led to SVR35 in 63% and TSS50 in 41% at Week 24, with a BMF improvement by ≥1 Grade in 28%, and a ≥20% reduction in JAK2 V617F VAF burden in 50% of patients. The randomized phase III TRANSFORM-1 study (NCT04472598) investigating navitoclax + ruxolitinib versus placebo + ruxolitinib in JAKi-naïve patients with intermediate-2 or high-risk MF is currently underway.
Navtemadlin1
Navtemadlin is an oral, mouse double minute 2 (MDM-2) inhibitor that upregulates p53 activity to induce apoptosis of TP53wt CD34+ MF cells. The phase I dose escalation study identified the recommended phase II expansion dose as 240 mg, administered for 7 days with 21 days off in a 28-day cycle.
Single-agent navtemadlin demonstrated a clinical benefit in patients with MF who were relapsed/refractory (R/R) to JAKi, significantly reducing spleen volume and improving symptoms. The median progression-free survival (PFS) and overall survival (OS) were 13.8 and 35.2 months, respectively. Across all cohorts, correlations were found between driver gene VAF reduction of ≥20% and PFS and OS, as well as normalization of CD34+ blood counts with PFS and OS. These results provided the rationale for the ongoing phase III BOREAS study (NCT03662126) investigating navtemadlin vs best available therapy in patients with MF who are R/R to JAKi. Navtemadlin plus ruxolitinib is also under investigation in this patient population, and results were summarized on the hub here.
Imetelstat1
Imetelstat is a telomerase inhibitor currently under investigation in the phase II IMbark trial (NCT02426086) in intermediate-2 or high-risk patients with MF who are R/R to JAKis. Patients were randomized to receive either 9.4 mg/kg or 4.7 mg/kg of imetelstat every 3 weeks. A greater clinical benefit was observed at the higher dose of imetelstat (Figure 1). Moreover, improved BMF and a ≥20% VAF reduction was associated with a longer median OS and higher survival rate.
Figure 1. Clinical benefit of imetelstat at the 4.7 vs 9.4mg/kg dose*
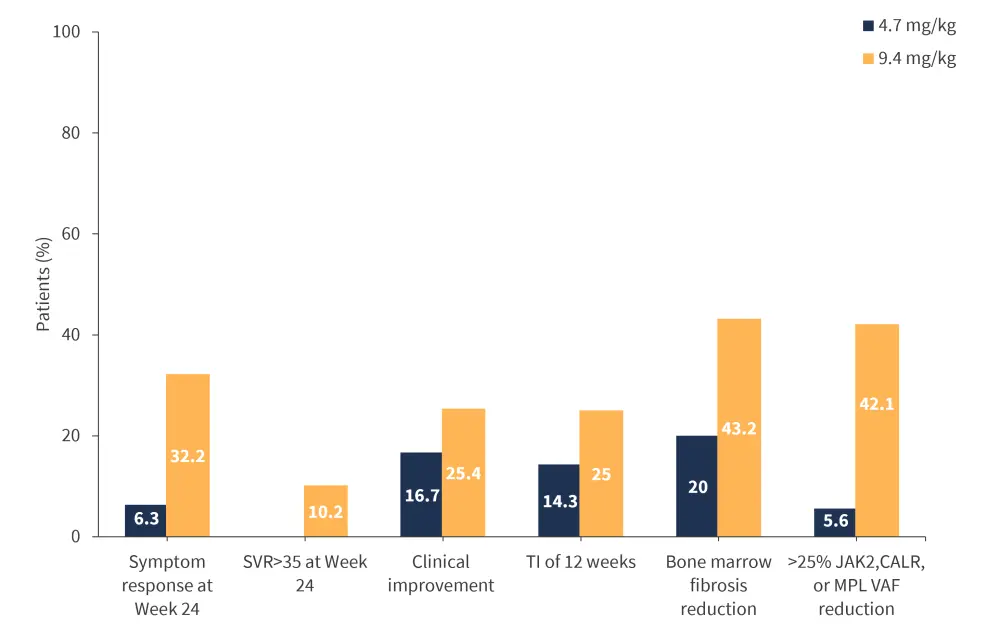
SVR, spleen volume reduction; TI, transfusion independence
*Data from Mascarenhas.1
Results from the phase II IMbark trial provided the rationale for the open-label, randomized phase III IMpactMF study (NCT04576156) evaluating imetelstat vs non JAKi best available therapy for patients with intermediate-2 or high-risk MF who were refractory to at least one JAKi treatment. The primary endpoint is OS; secondary endpoints include spleen and symptom response rate at Week 24, PFS, and safety.
Conclusion
This article highlights the promising efficacy of novel non-JAKi treatment for reducing spleen volume and improving symptoms for both JAKi-naïve patients and JAKi R/R patients with MF. Agents in late phase development include pelabresib (bromodomain and extra-terminal protein inhibitor), imetelstat (telomerase inhibitor), navtemadlin (MDM2i), and navitoclax (BCL2i). To date, none of these agents have shown disease course modification, this activity is under investigation in ongoing randomized phase III trials. Future research in this area should focus on identifying clinical and molecular biomarkers that can facilitate a personalized therapeutic approach. There will likely be a paradigm shift in the near future to incorporate combination therapies in frontline settings and non-JAK2i sequencing; this could translate to improved survival outcomes for MF patients.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

